The positive outlooks of studying negatively-charged chiral molecules
Promising analyses with photoelectron circular dichroism spectroscopy
From the difference in taste of spearmint and fennel, to the consequences of a drug, that either cures morning sickness or causes birth deformities, chirality and the properties of enantiomers, which trigger these differences, are known to be a crucial aspect of biological processes. Despite a pair of enantiomers’ distinct biological behavior, their similar chemical structures make them difficult to distinguish by analytical or spectroscopic methods. Much effort has gone into finding chiral discrimination techniques that can match nature’s ability to differentiate between increasingly small samples of two enantiomers.
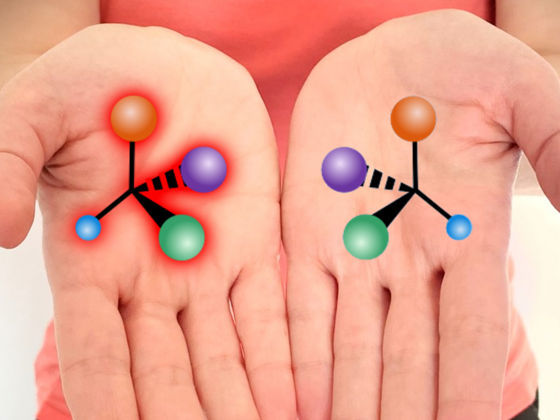
Chirality is defined as a pair of molecules, called enantiomers, which are non-superimposable mirror images of one another. Although these molecules look similar, they can have drastically different behavior in nature.
FHI
In the past two decades, a new wave of chiral discrimination techniques has emerged, which exhibit chiral sensitives that are magnitudes greater than their predecessors. This increased sensitivity allows for study of isolated chiral molecules in the gas-phase, at very low concentrations of initial sample. One of these contemporary techniques, which holds significant analytical promise, is known as photoelectron circular dichroism (PECD) spectroscopy. PECD is a chiral optical effect that emerges in the photoemission of electrons from a sample of chiral molecules, when illuminated with chiral light (ie. circularly polarized light). The effect is a difference in the average direction the electrons depart the molecular sample (either in the forward or backward direction). This direction is a function of the handedness of light and the enantiomer being probed. This effect is incredibly sensitive to many characteristics of a chiral molecule leading to an effect that is exceptionally perceptive to the chiral potential of a molecule.
Although studies of PECD have historically been carried out on neutral molecules, researchers at the Fritz Haber Institute have explored this effect in anions. The use of anions for PECD spectroscopy has a few important analytical advantages: First, as anions are charged particles, ion optics can be used for mass selection. Often, industrial chiral samples are known to be multi-component. The addition of mass selective capabilities would enable the isolation of target molecules before the photoemission process, thus simplifying final analysis. In addition, as this effect manifests through the removal of an electron from a chiral molecule, lower energies for electron detachment are preferential, as they allow for the use of commercially-available table-top lasers. Ionization of an electron from a closed-shell neutral molecule requires either high energy synchrotron radiation or a multiphoton process by visible or near-UV lasers. The smaller photon energies required for detachment of the extra electron in anions are accessible by common table-top lasers through single-photon detachment processes.
The research team in the Molecular Physics department has recorded a energy-resolved PECD signal for a mass-selected anion, for the first time. This is not only an important benchmark for the analytical possibilities of this technique, but also opens the door to understanding the differences in electron dynamics between photoionization of neutrals and photodetachment of anions. As the observation of this effect in anions has lagged two decades behind it’s observation in its neutral counterpart, comparisons of these photoemission processes could unlock comprehension of the universal dynamics that govern the PECD effect. In their initial findings, the research team has demonstrated a PECD effect surprisingly similar in magnitude to its neutral counterpart, and an effect that persists at much higher electron kinetic energies than was to be expected, when considering conventional theoretical descriptions of electron photodetachment. These results reveal a current gap in knowledge of this effect, which necessitates further investigation.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!