Powerhouses of the Cells: Mitochondria Have a Waste Disposal Mechanism to Get Rid of Mutated mtDNA
A research team has identified a molecular target that could open up new therapeutic options to treat aging-associated diseases
Scientists at the University of Cologne have discovered how cells can eliminate mutated mitochondrial DNA (mtDNA). mitochondria are the powerhouses of our cells. Due to their evolutionary descent from bacteria, they still have genetic material packaged in chromosome-like structures (nucleoids). They convert the chemical energy in our food into a biologically usable form. A team of researchers from the University of Cologne’s Physiology Centre at the Faculty of Medicine, the Centre for Molecular Medicine Cologne (CMMC) and the CECAD Cluster of Excellence for Aging Research has now shown that mutations of the mtDNA lead to a local rearrangement of proteins in the mitochondrial membrane. The mutated mtDNA is targeted, eliminated, and subjected to autophagy, the cellular ‘waste disposal’. The results have appeared in Nature Communications under the title ‘Mitochondrial membrane proteins and VPS35 orchestrate selective removal of mtDNA’.
Symbolic image
Computer-generated image
In many tissues, mutations in mtDNA accumulate as a result of normal aging. These kinds of mutations are an important cause of many aging-associated diseases. There are thousands of copies mtDNA in every cell, so mitochondrial function is only impaired when the percentage of mutated mtDNA molecules exceeds a certain threshold value. It has long been established that mitochondrial damage, including acute mtDNA damage, triggers the process of mitophagy. In this process, dysfunctional mitochondrial parts are selectively degraded and recycled.
Dr David Pla-Martin, the lead author of the current study, explained the details: ‘What is new in our study is that this mechanism does not affect the cells’ endowment with mitochondria, but only clears out the damaged mtDNA. By labelling neighbouring proteins – so-called proximity labelling – we showed that mtDNA damage leads to the recruitment of endosomes in close proximity to nucleoids.’ Their removal is coordinated by the interaction of the nucleoid protein Twinkle and the mitochondrial membrane proteins SAMM50 and ATAD3. ATAD3 controls their distribution, SAMM50 induces the release and transfer of the nucleoid to the so-called endosomes. ‘This additionally prevents the activation of an immune response. The protein VPS35, the main component of the retromer, mediates the maturation of early endosomes into late autophagy vesicles, where degradation and recycling ultimately take place,’ said Pla-Martin.
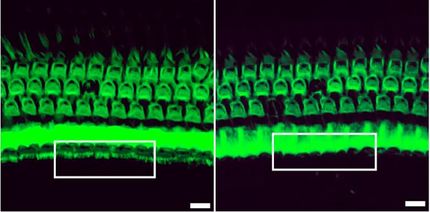
Using a mouse model in which mtDNA mutations lead to impaired muscle regeneration, the scientists also showed that selectively mutated mtDNA can be removed by stimulating the activity of the autophagy mechanism with rapamycin. The total number of mtDNA copies thus remains constant, preserving mitochondrial function.
Group leader Professor Dr Rudolf Wiesner added: ‘Mutations in the genes that code for these proteins lead to severe neurological diseases, in VPS35 for example Parkinson’s disease. We now want to use these proteins as new molecular targets to open up entirely new treatment options for these kinds of aging-associated diseases.’ Although the road to therapeutic application could still be long, the research team sees a promising approach here.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Chondromalacia_patellae
Fate Therapeutics Expands Stem Cell Modulator Pipeline with Acquisition of Verio Therapeutics
Sindicato da Indústria de Produtos Farmacêuticos no Estado de São Paulo (SINDUSFARMA) - São Paulo, SP, Brazil
Category:Acanthocephalans