Fighting Ovarian Cancer
Rhenium tricarbonyl complex with antitumor activity targets Fe-S cluster biogenesis
Metal-containing complexes have taken up center stage in the search for new cancer drugs that are as free of side effects as possible. In the journal Angewandte Chemie, a research team has now described how a very low dose of a rhenium metal complex interferes with cellular metabolism to such an extent that it kills ovarian cancer cells.
© Wiley-VCH
Cisplatin was the first metal-containing antitumor drug and others have been discovered since. Recently, a new potential antitumor drug was found in TRIP, a special rhenium carbonyl complex. TRIP causes rapid protein aggregation, which puts the endoplasmic reticulum (ER)—where protein synthesis, modification, and folding occur—under strain, hyperactivating the unfolded protein response (UPR). UPR is a cellular response to the accumulation of a large number of incorrectly folded proteins in the ER. This results in the programmed cell death (apoptosis) of tumor cells.
A team led by Samuel M. Meier-Menches at the University of Vienna (Austria) and Justin J. Wilson at Cornell University (Ithaca, NY/USA) has analyzed the effects of TRIP in more detail using chemoproteomics. This method is used to identify which cellular proteins are bound by a drug. Despite the broad toxic effects of TRIP, the team was able to identify 89 individual, dose-dependent, potential cellular target proteins in a line of ovarian cancer cells—this is called the target landscape for TRIP.
In addition, the team characterized the response of the living cancer cell line to treatment with TRIP by using proteome profiling. This involves comparing the proteome, which is all the proteins of the cells, with and without addition of various doses of the drug and noting differences in the concentrations of individual proteins.
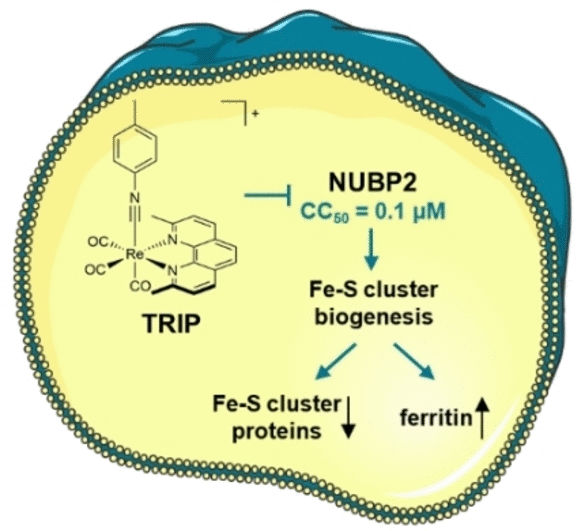
Taken together, these data point to the Fe-S cluster biogenesis factor NUBP2 as the probable starting point for the cellular processes triggered by TRIP. Fe-S clusters are complexes made of several iron and sulfur atoms. As cofactors, they play an important role in many enzymatic reactions, such as the respiratory chain. NUBP2 is essential for the production of proteins with Fe-S clusters. Treatment with TRIP significantly reduced the number of Fe-S proteins in ovarian cancer cells. The quantity of the iron storage protein ferritin increased drastically. The lack of Fe-S cluster proteins for cell respiration was substantiated by the bioenergetics of the tumor cells.
TRIP disrupts the biogenesis of Fe-S clusters in ovarian cancer cells in doses that are so small that they are not generally cytotoxic. This makes it an interesting starting point for development of a selective antitumor drug with few undesired side effects. This new combined research method could be used broadly to study the polypharmacology (effect on various targets) of metal-based drug candidates.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.