Nobel Prize in Chemistry 2022 for the development of click chemistry and bioorthogonal chemistry
Nobel Prize goes to a trio of researchers from the USA and Denmark
The Royal Swedish Academy of Sciences has decided to award the Nobel prize in chemistry 2022 to Carolyn R. Bertozzi (Stanford University, CA, USA), Morten Meldal (University of Copenhagen, Denmark) and K. Barry Sharpless (Scripps Research, La Jolla, CA, USA) “for the development of click chemistry and bioorthogonal chemistry”.
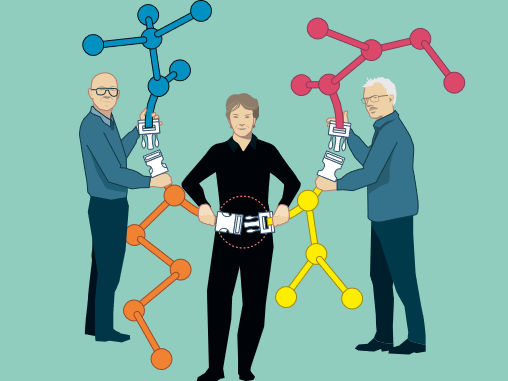
© Johan Jarnestad/The Royal Swedish Academy of Sciences
It just says click – and the molecules are coupled together
The Nobel Prize in Chemistry 2022 is about making difficult processes easier. Barry Sharpless and Morten Meldal have laid the foundation for a functional form of chemistry – click chemistry – in which molecular building blocks snap together quickly and efficiently. Carolyn Bertozzi has taken click chemistry to a new dimension and started utilising it in living organisms.
Chemists have long been driven by the desire to build increasingly complicated molecules. In pharmaceutical research, this has often involved artificially recreating natural molecules with medicinal properties. This has led to many admirable molecular constructions, but these are generally time consuming and very expensive to produce.
“This year’s Prize in Chemistry deals with not overcomplicating matters, instead working with what is easy and simple. Functional molecules can be built even by taking a straightforward route,” says Johan Åqvist, Chair of the Nobel Committee for Chemistry.
Barry Sharpless – who is now being awarded his second Nobel Prize in Chemistry – started the ball rolling. Around the year 2000, he coined the concept of click chemistry, which is a form of simple and reliable chemistry, where reactions occur quickly and unwanted by-products are avoided.
Shortly afterwards, Morten Meldal and Barry Sharpless – independently of each other – presented what is now the crown jewel of click chemistry: the copper catalysed azide-alkyne cycloaddition. This is an elegant and efficient chemical reaction that is now in widespread use. Among many other uses, it is utilised in the development of pharmaceuticals, for mapping DNA and creating materials that are more fit for purpose.
Carolyn Bertozzi took click chemistry to a new level. To map important but elusive biomolecules on the surface of cells – glycans – she developed click reactions that work inside living organisms. Her bioorthogonal reactions take place without disrupting the normal chemistry of the cell.
These reactions are now used globally to explore cells and track biological processes. Using bioorthogonal reactions, researchers have improved the targeting of cancer pharmaceuticals, which are now being tested in clinical trials.
Click chemistry and bioorthogonal reactions have taken chemistry into the era of functionalism. This is bringing the greatest benefit to humankind.
Most read news
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.