Making the Invisible Visible – Two-Step Mechanism Discovered that Enables Innate Immune System to Detect Viruses Like HIV Early
Understanding this mechanism could be helpful for the development of AIDS vaccines
Researchers at the Paul-Ehrlich-Institut participating in an international research network have discovered a two-step mechanism of the innate immune system that also makes it possible to specifically recognise HIV (human immunodeficiency virus) and trigger an early immune response. This knowledge could be used in the development of vaccines that could strengthen this mechanism. The body could thus produce its own defence against HIV effectively and early.
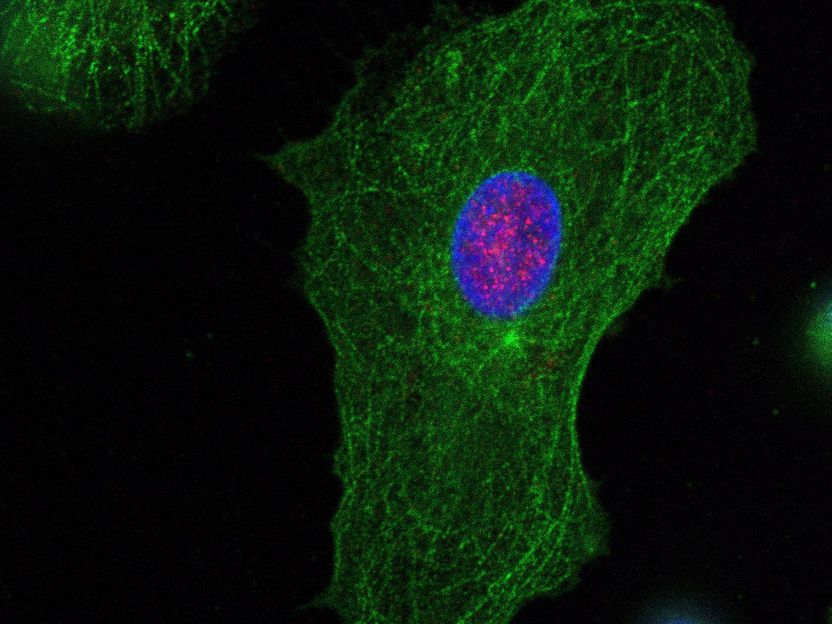
Monocyte-Derived Dendritic Cells (MDDCs) from a healthy donor stained for PQBP1 (red) and the cellular structural component tubulin-beta (green). The nuclear compartment is stained in blue.
N. Hein-Fuchs / Paul-Ehrlich-Institut
The human immunodeficiency virus HIV-1 belongs to the family of lentiviruses, which poses special challenges to medicine. HIV causes AIDS, a slowly progressing, chronic infectious disease, by circumventing the defence mechanisms of the immune system.
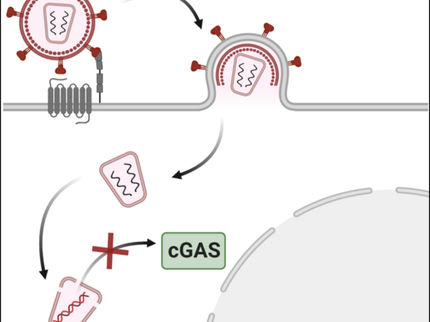
However, the immune system seems to have the ability to detect HIV at an early stage. Researchers from the Paul-Ehrlich-Institut took part in an international research network coordinated by Dr Chanda and Dr Yoh at the Sanford Burnham Prebys Medical Discovery Institute in La Jolla, California. Together with colleagues from New York, Chicago, Sydney and New Haven, they were able to gain new insights into the immune system. The research network was able to elucidate the mechanism by which the genetic information, the HIV virus DNA in the cell, can be recognised as foreign and thereby trigger a defence mechanism. The HIV virus is an RNA virus. After penetration into a cell, only a single DNA copy originating from the RNA genome is synthesised in the HIV virus and then integrated into the genome of the host. Thus, it is treated by the cell like a normal cell gene and remains invisible to the host defence system.
How can the cellular monitoring mechanism detect this small amount of DNA, which only occurs temporarily? The scientists identified a hitherto unknown two-step mechanism that makes this possible. In the first step, a receptor identified in 2015, the polyglutamine binding protein 1 (PQBP1), marks the intact viral capsid. The capsid is the protein structure used to package the viral genome. The already known DNA sensor protein, known as the cyclic GMP-AMP synthase (cGAS), is required in the second step. Here, the HIV virus converts its RNA into DNA during the reverse transcription process and the viral capsid begins to decompose.
This two-stage recognition of both pathogen protein (HIV capsid) and pathogen DNA (HIV DNA) ensures that the subsequent activation of immune signalling pathways (via interferon, IFN) is not spontaneously activated by only one component. This ensures that the body's own structures are not accidentally attacked. If activation occurs after this double detection, a powerful and targeted inflammatory reaction is mediated. This triggers a robust response to “true” foreign DNA species and at the same time bypasses an unwanted self-activation of host DNA.
The cooperative study not only offers new insights into the innate cellular detection of viruses from the lentivirus family, but also illustrates how sensors in the cells can be directed via additional factors and control mechanisms to specific and possibly rare or short-lived pathogen-associated molecular patterns (PAMPs) occurring in microbes. Other pathogens could also possibly be detected in a very targeted and efficient manner by following this concept.
Original publication
Yoh SM, Mamede JI, Lau D, Ahn N, Sánchez-Aparicio MT, Temple J, Tuckwell A, Fuchs NV, Cianci GC, Riva L, Curry H, Yin X, Gambut S, Simons LM, Hultquist JF, König R, Xiong Y, García-Sastre A, Böcking T, Hope TJ, Chanda SK (2022): Recognition of HIV-1 capsid by PQBP1 licenses an innate immune sensing of nascent HIV-1 DNA. Mol Cell Jul 8 [Epub ahead of print].