Evotec and Sernova announce exclusive strategic partnership for iPSC-based beta cell replacement therapy to advance a 'functional cure' for diabetes
Evotec SE and Sernova Corp., a clinical-stage company and leader in regenerative medicine cell therapeutics announced a partnership in the field of diabetes. Both Companies will leverage their respective strengths to develop an implantable iPSC-based beta cell replacement therapy for the treatment of insulin-dependent diabetes, including type 1 and 2.

Symbolic image
pixabay.com
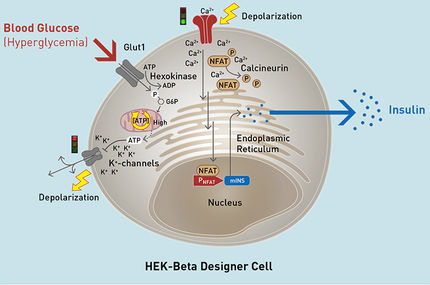
The partnership leverages iPSC-based beta cells from Evotec’s QRbeta initiative. Evotec reliably produces human iPSC-based beta cells in islet-like clusters in a quality controlled (“QC”) scalable bioreactor process. Those islet-like clusters are functionally equivalent to primary human islets in their ability to normalise blood glucose levels in in vivo models over several months.
Evotec’s iPSC-based beta cells will be combined with Sernova’s proprietary Cell Pouch™. In particular, it enables vascularisation of the cell implant and thus ensures long-term survival and optimal function in patients. The combination of primary donor islets and Cell Pouch has achieved long-lasting therapeutic results in patients enrolled in Sernova’s US-based Phase I/II clinical trial, including sustained insulin-independence in high-risk Type 1 Diabetes patients who previously required insulin injections for survival. Moreover, Sernova will evaluate local immune protection technology to protect non-modified beta cells and avoid the requirement for immunosuppressive treatment. The goal of the partnership is the development of an off-the-shelf iPSC-based beta cell replacement therapy device for the treatment of patients living with insulin-dependent diabetes.
Sernova has acquired an option for an exclusive global license to Evotec’s Induced Pluripotent Stem Cell (iPSC)-based Beta cells for use with its Cell Pouch system to treat diabetes. From an operational perspective, the pre-clinical development programme(s) will be jointly funded until IND acceptance. Sernova has the right to exercise its option for an exclusive global license upon IND filing. Evotec will contribute cell manufacturing through commercialisation and decide in the future on joint funding of clinical development. Upon commercialisation, there will be a profit-sharing arrangement between the two companies, with the split dependent upon Evotec’s participation in the clinical development programme.
In conjunction with the agreement, Evotec has committed to a strategic € 20 m equity investment in Sernova (approx. CAD$ 27 m at an €/CAD$ fx rate of 1.355).
Dr Cord Dohrmann, Chief Scientific Officer of Evotec, commented: “We searched long and hard for the right partner. Sernova clearly ticks all boxes with their clinically validated Cell Pouch™ technology, which fits perfectly to Evotec’s iPSC-based beta cells. Together we will progress a highly differentiated first-in-class beta cell therapy into clinical development with the common goal to bring a truly transformative therapy to insulin-dependent diabetic patients. The operational synergies of Evotec’s and Sernova’s technologies puts Sernova in position to become the world’s leader in beta cell replacement therapy. Our equity investment underlines our strategic interest in this collaboration with Sernova. We are very much looking forward to collaborate with them on the project as well as to be part of their Supervisory Board.”
Dr Philip Toleikis, President, and Chief Executive Officer of Sernova, commented: “In tandem with our current clinical islet cell programme, Sernova entered into multiple pharmaceutical research collaborations to identify the highest quality and most compatible iPSC cell technology, and validate the cells pre-clinically within our Cell Pouch System. Evotec is an iPSC powerhouse having dedicated many years and substantial resources to developing high quality and stable stem cell technologies for multiple therapeutic applications. In every sense, both as a global strategic partner and as an iPSC expert, Evotec has exceeded all our expectations and we welcome them to join our advisory board. Today’s announcement of this joint iPSC beta-cell partnership completes the three pillars of our diabetes cell therapy platform. Alongside our clinically validated Cell Pouch System and recently acquired conformal coating immune protection technology, this now establishes a total regenerative medicine cell therapy solution for insulin-dependent diabetes.”