Structure of key protein for cell division puzzles researchers
Researchers provide a first 3D snapshot of the CCAN protein complex and raise fundamental questions towards the creation of artificial chromosomes
Human cell division involves hundreds of proteins at its core. Knowing the 3D structure of these proteins is pivotal to understand how our genetic material is duplicated and passed through generations. The groups of Andrea Musacchio and Stefan Raunser at the Max Planck Institute of Molecular Physiology in Dortmund are now able to reveal the first detailed structure of a key protein complex for human cell division known as CCAN. By using cryo-electron microscopy, the researchers show important features of the complex’s 16 components and challenge previous assumptions about how the complex is able to recognize the centromere, a crucial region of chromosomes in cell division.
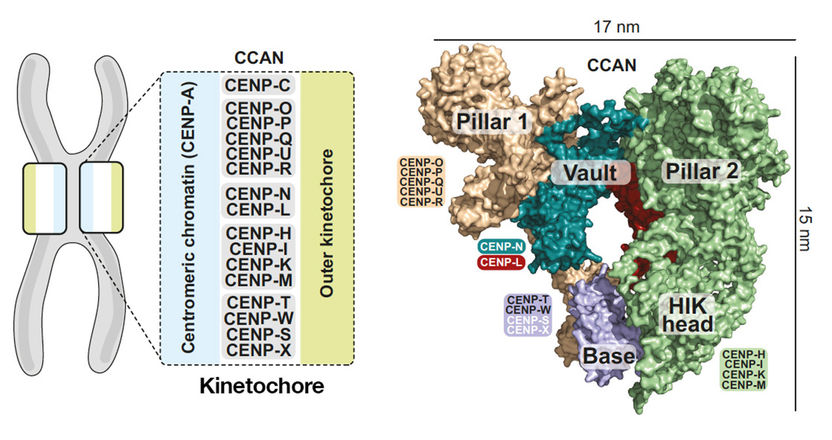
Organization of the human CCAN. Left: Scheme of the kinetochore organization with the CCAN subcomplexes binding to the centromere protein A (CENP-A). Right: Model of the surface of the CCAN’s 16 components organized in different subcomplexes.
MPI für molekulare Physiologie
At the centre of cell division
The centromere is a constriction in the chromosome, made of DNA and proteins. Most importantly, the centromere is the dock for the kinetochore, a machinery of about 100 proteins that drives the separation of two identical chromosomes during cell division and their delivery to the daughter cells. Previous research has shown that the kinetochore docks onto the centromere through the CCAN complex: The CCAN interacts with the centromere protein A, the landmark protein of the centromere. CCAN is also responsible for replenishing the centromere protein A once the cell division has taken place. Yet, the details of the interaction between CCAN and the centromere protein A remain elusive. “Understanding how CCAN recognises and binds to the centromere could potentially lead us to build a centromere from scratch”, says Musacchio. The centromere is a major hurdle for synthetic biologists who aim to engineer artificial chromosomes to restore missing functions or introduce new ones in cells.
Unresolved questions at the core
Scientists identified the CCAN complex over 15 years ago. “Yet, building up a pipeline to synthesize all proteins in vitro has been a major obstacle”, says Musacchio. After obtaining a first reconstitution of the human CCAN complex in vitro, Musacchio joined forces with Stefan Raunser, also at MPI Dortmund, who applied cryo-electron microscopy on the whole CCAN protein complex.
In the new publication, the MPI groups have been able to determine the 3D structural details of the human CCAN complex, highlighting its unique features and the implications for an interaction with the centromere protein A. “Contrary to what was expected, this structure does not directly recognise the centromere protein A in the standard configuration”, says Musacchio. The centromere protein A is most commonly packed with DNA and other proteins as a nucleosome, the standard unit of the genetic material. The authors are now suggesting that the centromere protein A may be embedded in the centromere with a different configuration that may facilitate the crucial interaction with CCAN. They plan to identify conditions that could lead to this new configuration and prove their hypothesis.
Original publication
Most read news
Original publication
Pesenti ME, Raisch T, Conti D, Walstein K, Hoffmann I, Vogt D, Prumbaum D, Vetter IR, Raunser S, Musacchio A. Structure of the human inner kinetochore CCAN complex and its significance for human centromere organization. Mol Cell. 2022 May 6:S1097-2765(22)00390-2.
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.





















































