How immune system cells recognize label that marks pathogens for destruction
A study by CIB-CSIC researchers describes at the atomic level how two key proteins interact and are recognized in the fight against infections
Scientists at the CSIC's Margarita Salas Biological Research Center (CIB-CSIC) have collaborated to explain at the atomic level how one of the most important mechanisms by which the immune system recognizes pathogens works. The study, published in Nature Communications, reveals how CR3 receptors on the surface of macrophages and other immune cells recognize the iC3b molecule with which the complement system labels pathogens to be destroyed by phagocytosis. This finding may contribute to the development of strategies against autoimmune and inflammatory diseases.
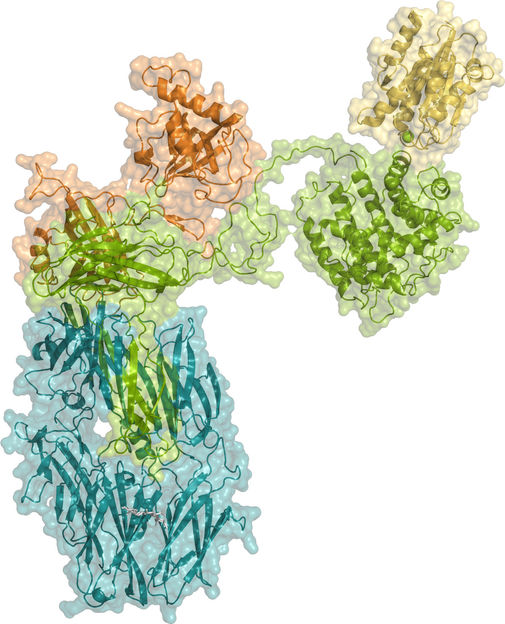
Crystallographic structure of iC3b in complex with the αI domain of CR3.
CIB-CSIC
The complement system is a fundamental component of innate immunity in humans. It consists of more than 30 proteins in plasma, plus receptors and regulators on the membrane of many cells, and is activated by the presence of pathogens and other substances that it recognizes as foreign. "It is our first defense against infection by pathogens and its mission is to tag pathogens with molecular signals for their destruction," says CIB-CSIC researcher M. Cristina Vega, lead author of the study.
When complement is activated on the surface of pathogens, the C3 protein, one of the most abundant proteins in plasma, undergoes a series of transformations that lead to the covalent attachment of thousands of activated C3 molecules to the surface of the pathogen (opsonization). The main binding protein or opsonin is iC3b, one of the activated C3 molecules.
Opsonization is essential for fighting infection, as the effector cells of the immune system are able to recognize iC3b-opsonized pathogens as a threat, attaching to them and phagocytizing them. To this end, macrophages employ a complex protein present on their surface known as complement receptor 3 (CR3). The interaction between iC3b and CR3 is therefore responsible for the adhesion and phagocytosis of iC3b-coated pathogens by macrophages. In the work presented by the CIB-CSIC researchers, the molecular details of how this interaction between iC3b and CR3 occurs are revealed.
"With this work we have discovered that the interaction of iC3b and CR3 proteins is modular in nature. This modularity confers great versatility and specificity to the interaction, favoring pathogen recognition in areas with a high density of iC3b molecules. Malfunction of these proteins can lead to increased susceptibility to infections and rare autoimmune diseases," explains Vega.
Although the general function of iC3b was known, its atomic structure as well as the details of iC3b specificity and recognition by CR3 were unknown. This new study completes the information on these two key infection-fighting proteins. "The resolution of the structure of this complex represents an important step in understanding how the cells of the immune system harness the activity of the complement system to detect and destroy pathogens, a fundamental process for preserving health against many infectious agents," says Francisco J. Fernández, first author of the study.
"The work highlights the importance of maintaining a continuous supply of iC3b molecules to the surface of pathogens by the complement system, which explains previous data that we did not fully understand and raises the possibility of intervening in the complement system to favor this process," explains Santiago Rodríguez de Córdoba, research professor at the CIB-CSIC and collaborator in this work.
Vega's and Rodríguez de Córdoba's groups belong to the CSIC Global Health Platform, where they collaborate on studies aimed at treating the most severe symptoms of covid-19 using strategies focused on the complement system. The two groups have previously described new mechanisms of immunoevasion in Streptococcus pyogenes and other pathogenic bacteria that interfere with the role of the complement protein C5a, reducing the attraction of neutrophils to sites of infection. The work they now publish will facilitate the development of strategies to modulate CR3 activity in autoimmune and inflammatory diseases.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in Spanish can be found here.
Original publication
Fernández FJ, Santos-López J, Martínez-Barricarte R, Querol-García J, Martín-Merinero H, Navas-Yuste S, Savko M, Shepard WE, Rodríguez de Córdoba S y Vega MC.; "The crystal structure of iC3b-CR3 αI reveals a modular recognition of the main opsonin iC3b by the CR3 integrin receptor."; Nature Communications.