Lockdown for tumour cells
Novel inhibitor locks tumour cells in place
Protein phosphatases are the counterplayers of protein kinases and together, these two groups of enzymes control virtually every aspect of cellular behaviour. Therefore, being able to modulate the activity of specific kinases or phosphatases is the basis for a growing list of medical treatments, from dampening inflammation upon organ transplantation to stopping the proliferation of leukemia cells. A novel inhibitor, discovered at the University of Konstanz, stalls a critical enzyme inside tumour cells, thereby locking the cells in place. This cellular lockdown stops tumour cells from invading healthy neighbouring tissue, thus potentially paving the way for the suppression of tumour metastasis. The findings were published in the online edition of “Cell Chemical Biology” on 19 April 2022.
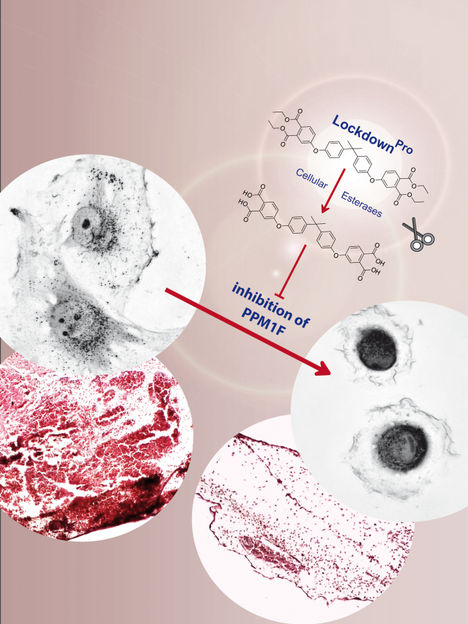
By selective inhibition of the protein phosphatase PPM1F, LockdownPro stalls tumor cell invasion
Tanja Grimm und Marleen Herbinger, Universität Konstanz
All cellular processes in our body, including growth, proliferation, differentiation and migration, depend on the interplay between protein kinases and protein phosphatases, enzymes that regulate the phosphorylation state and, thus, the function of proteins. Whenever the phosphorylation of key proteins runs out of control, cells suffer severe consequences. Not surprisingly, disregulated protein phosphorylation is one of the hallmarks of cancer cells.
Accordingly, small molecule inhibitors of these enzymes, most prominently kinase inhibitors, have revolutionized tumour treatment. In principle, phosphatases also constitute promising drug targets, however, this group of enzymes has been hard to tackle using small molecules. Now a research team led by Christof Hauck, professor of Cell Biology, has identified a specific inhibitor of the protein phosphatase PPM1F. As this enzyme is a key facilitator of cell motility, cells treated with the inhibitor are unable to move, resulting in tumour cells remaining stuck in place.
So far, developing specific inhibitors for protein phosphatases has been a challenge for biochemists. This is at least partly due to the shallow and unpretentious reactive centre of most protein phosphatases, which does not provide a prominent binding interface for inhibitors. Therefore, the best chance of blocking the activity of a specific phosphatase comes from inhibitors, which influence the enzyme from a distance, so called allosteric inhibitors. However, finding such an agent is difficult, like the search for the proverbial needle in the haystack.
Pushing chance by upscaling numbers
To find a compound with these peculiar properties, Tanja Grimm, a doctoral researcher in Professor Hauck's team and a member of the Konstanz Research School Chemical Biology, teamed up with the Screening Centre in the Department of Biology. The goal was to move from laborious biochemical tests of enzyme activity to an automated procedure on the microscale. Tanja Grimm and the Screening Centre were indeed able to test more than 55000 compounds, develop a chemi-informatic algorithm to identify the primary hit substances, re-evaluate 200 candidates multiple times, and afterwards end up with a handful of potential PPM1F inhibitors.
Though these molecules were all able to block the purified enzyme, only one compound was selective for PPM1F, while the rest exhibited undesired inhibitory activity for other enzymes. Unfortunately, the selective PPM1F inhibitor also had a drawback: It had polar groups, which made a passage through the cell’s membrane problematic, so the inhibitor could hardly reach the target enzyme in the cell's interior. To address this problem, Tanja Grimm partnered with a colleague from the Department of Chemistry to synthesize a modified version of the compound, which she then could apply to intact cells.
Stopping tumour cells on the spot
As PPM1F is often overexpressed in malignant tumours, the clear next step was to test the activity of the PPM1F inhibitor in human cancer cells. Previously, it has been shown that deletion of the PPM1F gene (by corrupting the protein-encoding DNA sequence) leads to immobile cells. Indeed, application of the inhibitor caused a similar phenotype: The tumour cells firmly stuck to the substrate and stopped moving. In light of this striking property, the researchers re-named their novel inhibitor "Lockdown", as it essentially blocked the high motility of these tumour cells.
The team then tested whether Lockdown would also stop tumour cells from migrating through tissue barriers, a process called tumour cell invasion. Tumour cell invasion is linked to the ability of tumour cells to form metastases at distant sites in the body, which is one of the key characteristics of malignant cells. Importantly, the application of Lockdown was able to stop human glioblastoma cells, a highly invasive form of brain tumour cells, from crossing tissue barriers and from entering healthy tissue. Accordingly, substances such as Lockdown could serve as the starting point for the development of metastasis-blocking agents.
PPM1F substrates are critical for tumour cell invasion
The possibility to control PPM1F via chemical inhibition now also opens a novel avenue for studying this interesting enzyme. Apparently, the substrates of PPM1F must be critical regulators of tumour cell motility and invasion. The researchers now hope that their work will enable the decoding of all these downstream substrates in order to identify additional targets for future metastasis blockers. "It might still be a distant dream, but being able to stop tumour metastasis would mean major progress, especially in situations, where the primary tumour can be surgically removed, but where the appearance of metastases would otherwise hang like a sword of Damocles over the patients," says Tanja Grimm, first author of the study.