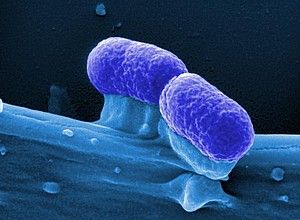
Bacterial shackle: Motility-inhibiting active substances against the gastric germ Helicobacter pylori
supplement or alternative to conventional antibiotic treatment
Helicobacter pylori, one of the most widespread bacterial pathogens, is responsible for hundreds of thousands of cases of stomach ulcers and stomach cancer worldwide every year. Due to increasing resistance of the bacterium to currently available therapeutically used antibiotics, new active agents are urgently needed. A group of scientists at the Ludwig Maximilian University of Munich (LMU) and the German Center for Infection Research (DZIF) has succeeded in identifying substances that could inhibit the bacteria’s motility and thus prevent it from multiplying and exerting its pathogenic effect.
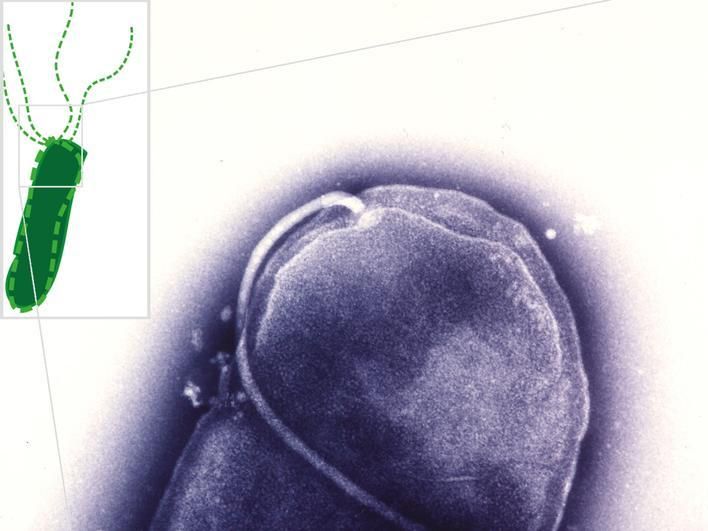
Electron microscopic image showing a flagellum—a bacterial propeller—attached to one end of a rod-shaped Helicobacter pylori bacterium (violet). Inset: Schematic of a bacterial cell with propeller flagella. The newly identified substances are able to inhibit the construction and rotation of the bacterial propellers.
© Christine Josenhans and Shin-Ichi Aizawa, LMU
‘The ability to move in the viscous environment of gastric mucus is essential for H. pylori to survive and multiply’, explains Professor Christine Josenhans, scientist at LMU’s Max von Pettenkofer Institute and the DZIF. The researchers are now trying to exploit this ability of the bacterium to develop alternative therapies.
In collaboration with scientists at the Helmholtz Centre for Infection Research in Braunschweig, the Hanover Medical School and the Charité University Hospital in Berlin, they tested almost 4,000 chemical substances for their potential effect on the movement mechanism of the bacteria. At one end of the bacterial cell, H. pylori carries a bundle of rotating flagella that act like propellers, helping the bacteria to move around in the stomach mucus. With a specifically designed screening procedure, the scientists were able to identify several substances that inhibit the structure of these bacterial propellers and virtually put a shackle on the bacterium.
For one of the substances, they were able to observe a strong reduction in bacterial proliferation in the stomach of mice infected with H. pylori, without significantly damaging the normal bacterial intestinal flora. ‘This would be a great advantage over conventional antibiotics, which also oftentimes permanently attack the essential “good” intestinal bacteria’, explains Prof. Josenhans, the senior author of the study.
Currently several classes of antibiotics need to be combined for successful treatment of an H. pylori infection, which however can lead to severe side effects and further increases antibiotic resistance of the bacteria. ‘The substances we call antimotilins could be a supplement or alternative to conventional antibiotic treatment options. In the long term, they could also help reduce the development of antibiotic resistance’, emphasises Prof. Sebastian Suerbaum, Chair of Medical Microbiology at the Max von Pettenkofer Institute and lead author of the publication.
Prof. Josenhans adds that the next step is to identify the exact mode of action of the active substances and to develop them further as new antibacterial treatment.
Original publication
Sebastian Suerbaum, Nina Coombs, Lubna Patel, Dimitri Pscheniza, Katharina Rox, Christine Falk, Achim D. Gruber, Olivia Kershaw, Patrick Chhatwal, Mark Brönstrup, Ursula Bilitewski, Christine Josenhans; "Identification of antimotilins, novel inhibitors of Helicobacter pylori flagellar motility that inhibit stomach colonization in a mouse model"; mBio; 2022