A new dimension in Stem Cell Signaling
Molecular morse code in stem cells encrypting differentiation information revealed
Divide, differentiate or die? Making decisions at the right time and place is what defines a cell’s behavior and is particularly critical for stem cells of an developing organisms. Decision making relies on how information is processed by networks of signaling proteins. The teams around Christian Schröter from the Max Planck Institute of Molecular Physiology in Dortmund and Luis Morelli from IBioBa have now revealed for the first time, that ERK, a key player in stem cell signaling processes information through fast activity pulses. The duration of the pulsing interval, might encode information essential for divergent fate decision in stem cell cultures.
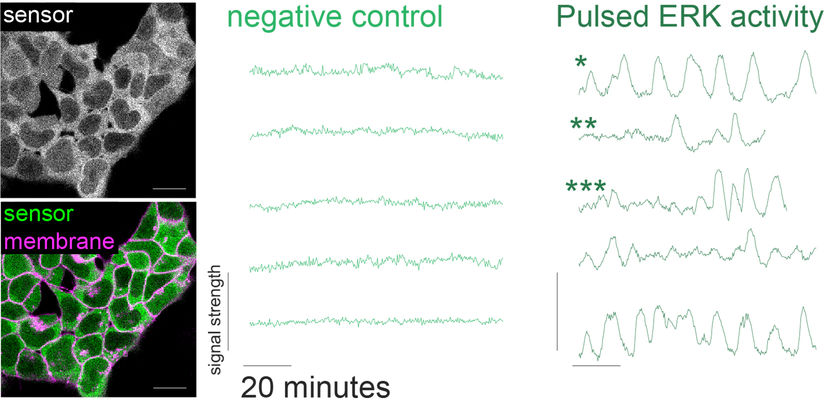
Pulsating ERK activity in stem cells. Left: sensor (green) in cells. Right: pulsating activity, light green curves are control and dark green curves the oscillations. *: regular pulses, **: isolated pulses, ***: pulsating and non-pulsating phases.
MPI of Molecular Physiology
During their development into the later embryo, stem cells go through a series of developmental steps. The transition between those steps is controlled by signaling molecules that are exchanged between neighboring cells. One of the most critical signals during early mammalian embryogenesis is the fibroblast growth factor 4 (FGF4). When it is recognized by a cell, this information is processed by a network of signaling proteins, resulting in a cellular response. The key players of the network, their role and interactions are by now well known, however only little is known about the signaling dynamics. But what does dynamics actually mean, and why are dynamics important?
Dynamics determine cell fate
In the posterchild example for the importance of dynamics in signal transduction, two different molecular signals trigger different cellular responses - differentiation and cell growth – even though they use the same signal transduction network. This is possible because the dynamics with which the signal transduction system is activated are specific for each of the two molecular signals: Whereas one activates the system for a short time leading to cell growth, the other activates the same system for a long time resulting in differentiation. Thus, signaling dynamics are clearly important to determine a cell’s fate. However, many studies so far could only look at fairly slow dynamics that unfolded over hours and that were the same in all cells; they were blind to fast dynamics, especially if these were different between stem cells in the same dish.
ERK activity pulses every six to seven minutes
The teams around Christian Schröter and Luis Morelli were now able to gain a better understanding of the fast signaling dynamics in stem cells. By introducing a fluorescent sensor in living stem cells, the scientists could measure the activity of the major signaling protein ERK in real-time. ERK activity is important for translating molecular signals into a genetic response and thus for regulating stem cell differentiation. “Measuring ERK activity in single stem cells at short timescale is experimentally very demanding and was never done in such a way before. For the first time, we could observe, that ERK activity pulses every six to seven minutes, faster than similar signals previously shown in other cell systems. In single cells, the pulses occurred often very regularly one after the other, but pulsing patterns were strikingly different between individual cells”, Christian Schröter says. The researchers could also observe, that with increasing FGF4 signal, the number of pulses increases when summing up over many cells, even though the durations of single pulses did not change with FGF4.
Interdisciplinary approach – Intercontinental collaboration
“This kind of data and its role on cell signaling is very hard to interpret. And that is the point, where our expertise kicked in”, Luis Morelli says, longstanding collaboration partner and group leader at the IbioBa, a partner Institute of the Max Planck Society. “We had to develop a new theoretical approach to describe the dynamics in time series. By doing this, we saw that the duration of the pulsing interval might encode information, since we could find pulses and silence. We call this new dynamic feature intermittent oscillations”.
“Oscillations are a more and more recognized feature of signaling processes. We hypothesize that the intermittent oscillations we found in stem cells work like a kind of morse code that encodes differentiation information. Presumably, it is the switch from pulsing to silence that plays a decisive role. The question is now, what do the dynamics tell us about the organization of signaling in stem cells? How are cells able to read the oscillations, and how do they affect the cell’s behavior? I am convinced that close collaboration between experimentalists and theorists is required to unravel the origins and functions of this new dimension in stem cell biology one day”, Christian Schröter says.



















































