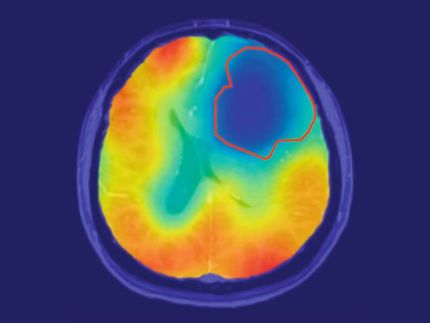
Experimental combinatorial therapy eliminates an incurable brain tumor
A study recently accepted for publication in The Journal of Clinical Investigation describes a new and effective therapy to treat glioblastoma: the concomitant use of ADI-PEG20 together with focal brain radiotherapy. This double treatment completely eliminated the tumour in the animal models used in the study. This study was carried out by researchers from the Institute of Biomedicine of Seville: Dr. Manuel Sarmiento Soto, Dr. Juan García Revilla, and Dr. José Luis Venero, in collaboration with Dr. Nabil Hajji and Dr. Nel Syed of Imperial College London.
Symbolic image
Milad Fakurian/Unsplash
Currently, glioblastoma is a terminal disease whose average life expectancy is less than 2 years. The treatments used at present are based on therapies that are more than 30 years old as no significant advances have been made to efficiently combat this type of tumour.
The results of this study offer a new therapeutic option against glioblastoma: the use of the drug ADI-PEG20, which eliminates systemic arginine, in combination with the application of focal brain radiotherapy. With this, it was possible to observe how a brain tumour, incurable to date, was completely eliminated in in vivo models that died of natural causes without showing any manifestation of the disease.
"With this new treatment, we were able to cure animals of an aggressive and terminal disease," says Dr. Sarmiento, "the post-mortem analyses detected how the cerebral immune response, and fundamentally the microglia cells, were activated during the treatment, directing their attack against the tumour cells and thus facilitating the tumour's complete elimination".
The data obtained are a ray of hope for the treatment of patients with this type of tumour, since, regardless of the success of the new anticancer drugs, their efficacy in the central nervous system is currently very limited, largely due to their difficulty in reaching the cancer cells located inside the brain.
Worth noting is another clinical trial (NCT04587830) also focused on another specific type of glioblastoma patient, studying the use of the same drug and whose results are proving to be very promising. Importantly, the drug did not manifest side effects in the patients involved in said trial.
This work has been funded both by Projects of the Ministry of Science and Innovation (RTI2018-098645-B-I00) and the Ministry of Economy and Knowledge of the Andalusia Regional Government (P18-RT-1372), as well as by the Brain Tumor Research, a UK charity focused on finding a cure for all types of brain tumours.
The research team firmly hopes to begin recruiting the first patients for the start of the Phase I clinical trial in the coming months.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.