Last line of defense: How bacterial populations are protected against viral infections
Bacterial dynamin protein is involved in a previously unknown protective mechanism against bacteriophage infections
Advertisement
bacteria as unicellular organisms are attacked by specialised viruses, the so-called bacteriophages or phages for short. To defend themselves against phage infections, bacteria feature a multi-stage protective mechanism, which in principle consists of components of an innate and an acquired immune system, just as in more complex organisms. It can interrupt the course of an infection at various points, for example by preventing the binding of a phage to the cell surface and the injection of phage DNA into the cell, by specifically removing foreign genetic information or by preventing the phage from replicating in the cell.
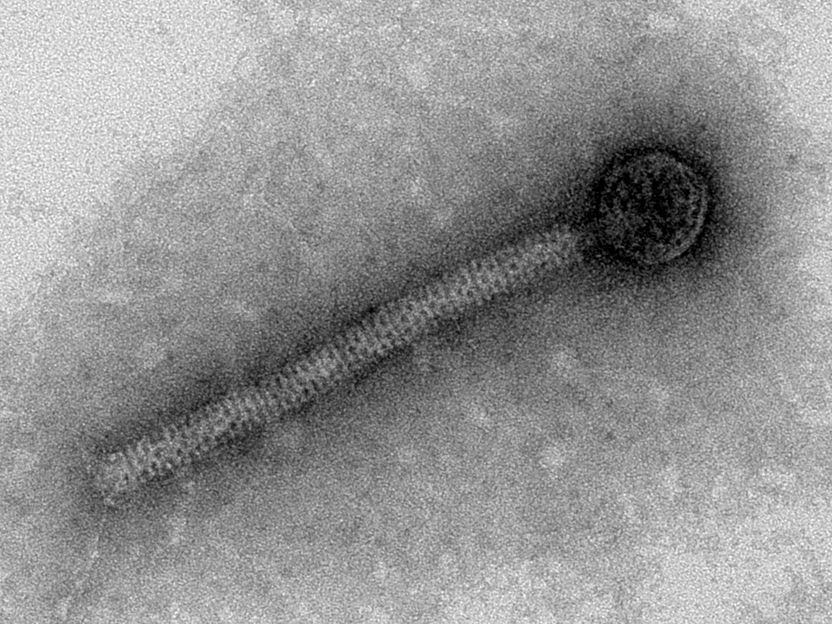
Transmission-electron micrograph of a bacteriophage: These bacteria-specific viruses first replicate their DNA in infected bacterial cells, then self-assemble their progeny from specific proteins and release them after the host cells dissolves.
© Dr Urška Repnik, Central Microscopy, Kiel University
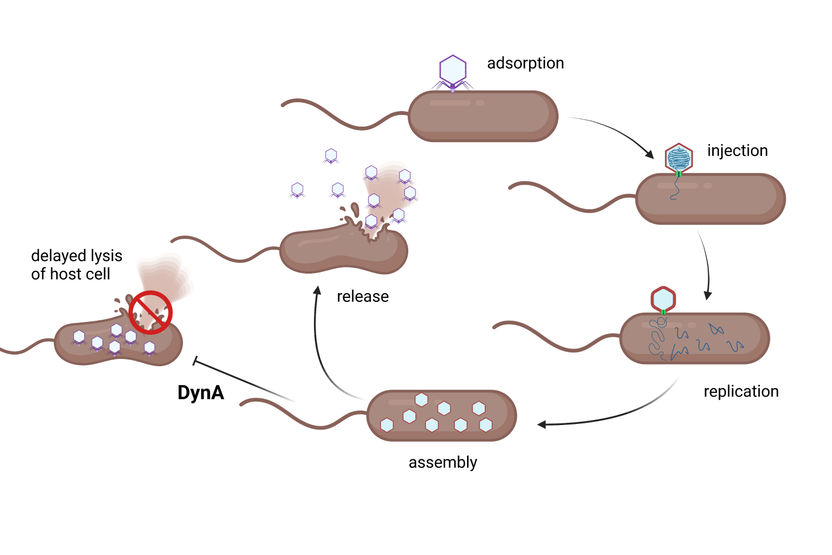
Bacterial dynamin proteins intervene at the end of the infection cycle and help to significantly slow down cell lysis, thus reducing the release of phage progeny.
© Prof. Marc Bramkamp
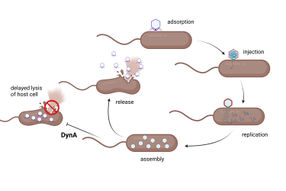
A research team from Kiel University has now succeeded in identifying an additional protective mechanism in this cascade of bacterial defence. The scientists from the Microbial Biochemistry and Cell Biology group led by Professor Marc Bramkamp investigated proteins from the so-called dynamin family, whose involvement in various processes in the cell membranes of multicellular organisms was already known. The Kiel researchers found out that dynamin also plays an important role in bacteria's defence against infections: By maintaining an intact cell membrane, the proteins prevent bacterial cells from bursting and thus explosively distributing the phages they contain. They thus add another barrier to the bacteria's protection against infection, which can inhibit the spread of viruses in the bacterial population despite already infected cells. The Kiel researchers published their new research results together with colleagues from the University of Marburg in the journal mBio.
Dynamin fuses cell membranes
Dynamin proteins are involved in numerous cellular processes in multicellular organisms that are mainly centred around interactions with membranes. These include, for example, the constriction of small membrane components, the vesicles, which in multicellular organisms transport messenger substances between cells of the nervous system. A few years ago, Bramkamp's research group was able to prove that dynamins also occur in unicellular organisms such as bacteria, where they are able to hold membrane components together by forming so-called oligomers. "Why bacteria possess this ability was previously unknown. In multicellular organisms, however, similar proteins are involved in combating viral infections, so we suspected they might also play a role in bacterial immune defence," explains Bramkamp.
If bacteriophages meet a bacterial population, it depends on the type of phage how the infection proceeds. Some pathogens penetrate the bacteria and incorporate their genetic material into the chromosome of the host. However, the phages only multiply and thus have harmful consequences for the bacterial cells when the host organism is stressed. This mode also occurs with numerous viruses that infect humans and only trigger disease symptoms when the immune system is weakened. Other pathogens, the so-called lytic bacteriophages, on the other hand, cause an infection cycle that is designed for direct reproduction and spread. If the bacterial cell does not succeed in interrupting this course, the infection ends with its destruction: a phage protein perforates the cell membrane, which leads to bursting and subsequent spreading of the phages, which then cause numerous further infections.
Last line of defence against phage infections
To clarify the question of whether dynamin is also related to virus defence in bacteria, the Kiel researchers conducted experiments with the bacterial model organism Bacillus subtilis. They investigated whether the presence or absence of dynamin in the bacterial cells has an effect on the course of a phage infection. To do this, they experimentally created different bacterial cultures in which dynamin was either absent or increased. "It turned out that when the dynamin was absent, the cultures became very sensitive to phage infections and they became infected much more quickly and effectively. On the opposite, cells with upregulated dynamin showed almost complete resistance to phage infections," Bramkamp underlines.
To explain the functional connection, the researchers observed the infection mechanism and the role of dynamin therein step by step. "The cycle initially runs normally from the attachment of the phage to its multiplication inside the cell. In fact, however, the dynamin then appears at the very end related to an additional resistance mechanism. It ensures that the infected cells lyse with a delay, i.e. are destroyed more slowly and, above all, do not burst explosively," says Bramkamp. The researchers were able to confirm this effect using imaging techniques: Fluorescence-stained cells showed that cell integrity is maintained longer in the presence of dynamin. "In addition, we were able to observe how dynamin proteins collect at the cell membrane. The protein is no longer evenly and dynamically distributed in the bacterial cell after infection. Instead, it gathers at the position where the cell membrane is attacked and significantly delays its rupture," explains co-author Peter Graumann, Professor for Biochemistry at Marburg University.
Although the presence of dynamin cannot prevent infected bacterial cells from being destroyed, the mechanism still effectively counteracts infection: By preventing explosive spread, phage distribution in the population is hindered and the infection thus spreads more slowly; at the same time, the additional stages of bacterial phage defence can take effect again in the overall population. The protective effect of the dynamin acts non-specific and includes lytic and non-lytic phage species equally. "This creates a last line of defence that complements other resistance mechanisms earlier in the infection cycle. At the population level, the bacteria thus become significantly less sensitive to phage infections, which means an important evolutionary advantage," summarises Bramkamp.