Cannabinoids from amoebae
New process for the production of active compounds
A research team at the Leibniz Institute for Natural Product Research and Infection Biology - Hans Knöll Institute (Leibniz-HKI) in Jena, Germany has developed a new method to produce complex natural products in amoebae. These polyketides include various antibiotics but also olivetolic acid, a precursor of the herbal active ingredient tetrahydrocannabinol (THC). The results were published in Nature Biotechnology.

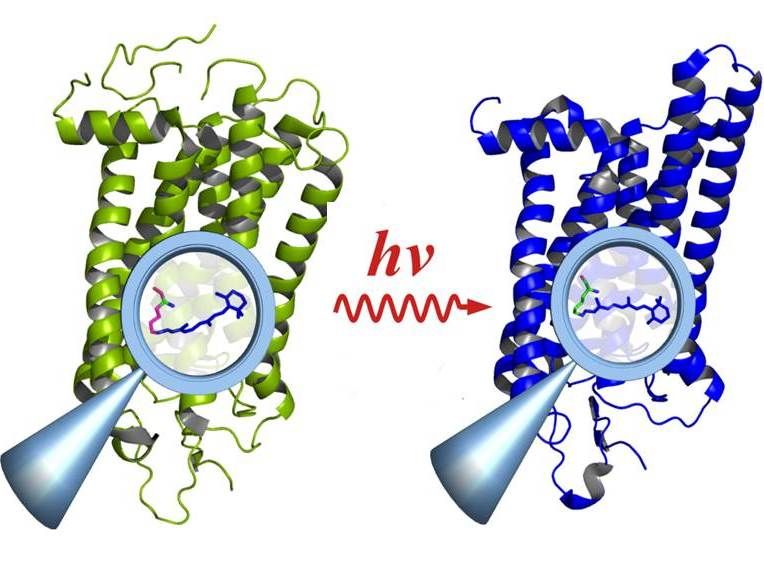
The combination of an enzyme from the cannabis plant with an enzyme from the amoeba D. discoideum enables the biotechnological production of a key cannabinoid precursor.
Falk Hillmann/Leibniz-HKI
Polyketides are natural products with a wide range of therapeutic applications. Among them are dietary supplements, various antibiotics such as erythromycin, and one of the key cannabinoid precursors: Olivetolic acid. It is needed for the synthesis of tetrahydrocannabinol (THC). The medical use of this psychoactive substance is being intensely researched, and it is already being used to provide relief for patients with neurological diseases and pain, among other things.
THC is a natural ingredient in the cannabis plant. "However, isolating THC in its pure form from the abundance of substances is very complex," says Falk Hillmann, head of the junior research group "Evolution of Microbial Interactions" at Leibniz-HKI and coleader of the study. Chemical synthesis of THC on the other hand is expensive and the yield is low. That's why he and a team are researching how such plant substances can be efficiently produced biotechnologically.
"So far, bacteria such as Escherichia coli or the yeast Saccharomyces cerevisiae are mainly used, but neither of them are native producers of natural products," explains Vito Valiante, head of the cooperating junior research group "Biobricks of Microbial Natural Product Syntheses" at the Leibniz-HKI. Accordingly, a large number of genetic modifications is necessary to enable synthesis in these classic model organisms. The research team is thus looking for alternatives. One promising candidate is the amoeba Dictyostelium discoideum, which already possesses numerous biosynthetic genes for the production of natural products such as polyketides. "Taking a closer look at the genes, we noticed that some show a high similarity to plant biosynthetic genes," said first author Christin Reimer, who is working on the topic for her PhD.
To test how well D. discoideum is suited as a chassis organism for biotechnological production, the researchers first had the amoeba produce the food supplement resveratrol, also a polyketide. Afterwards they incorporated the plant enzyme that produces the THC precursor olivetolic acid into the amoeba's genome. However, the addition of chemical precursors was still necessary to enable the synthesis.
To circumvent this, the researchers took advantage of the amoeba's natural properties and combined the plant enzyme with an amoebic enzyme. "The amoeba is able to produce the required precursor, a hexane unit, directly on site," Hillmann explains. Thus, the research team succeeded in producing a functional inter-kingdom hybrid enzyme that produces olivetolic acid without any further additives.
"Through our research, we have shown that the amoeba Dictyostelium can be used as a biotechnological production platform for polyketide-based natural products," says Reimer. The researchers already filed a patent for the process, and are striving to improve it on an ongoing basis. "Our next goal is to insert the two enzymes that are still missing in order to be able to produce the final product THC in the amoebae," Hillmann says.
Moreover, a team from the Bio Pilot Plant at the Leibniz-HKI was also involved in the research. Johann Kufs, joint first author of the study alongside Reimer, is in charge of developing and optimizing the synthesis process for industrial application.