Optimization of mRNA containing nanoparticles
Investigations at FRM II aid in the development of mRNA medications
The research neutron source Hein Maier-Leibnitz (FRM II) at the Technical University of Munich (TUM) is playing an important role in the investigation of mRNA nanoparticles similar to the ones used in the Covid-19 vaccines from vendors BioNTech and Pfizer. Researchers at the Heinz Maier-Leibnitz Zentrum (MLZ) used the high neutron flux available in Garching to characterize various formulations for the mRNA vaccine and thus to lay the groundwork for improving the vaccine's efficacy.
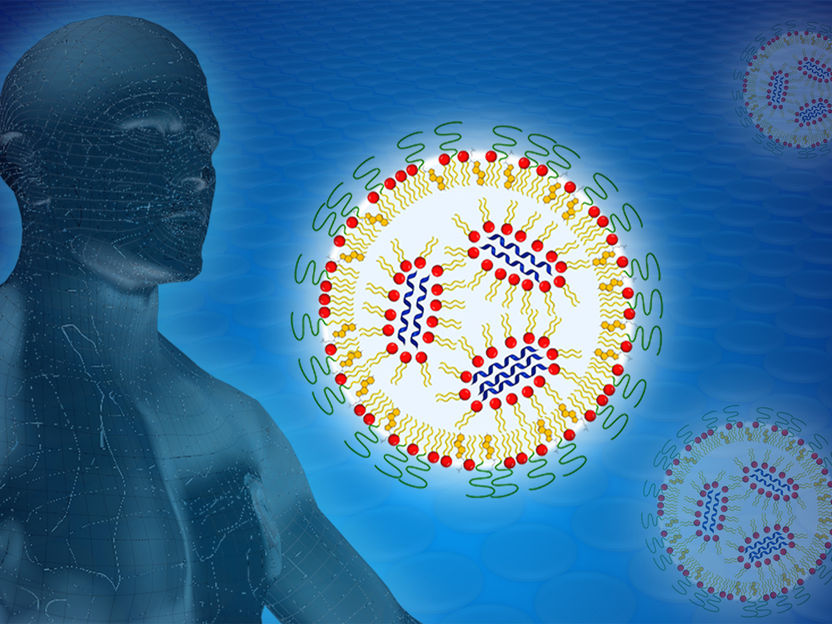
To protect it on the way to the cell, the mRNA (blue) is packaged in a lipid nanoparticle. With the help of neutrons from the research neutron source of the Technical University of Munich, a research team examined various formulations in order to optimize the transfer of the mRNA into the cell.
BioNTech / Reiner Müller / TUM / FRM II
The idea of using messenger RNA (mRNA) as an active ingredient is a brilliant one: The molecule contains the specific blueprint for proteins which are then synthesize by the cell. This makes it generally possible to provide a very wide spectrum of different therapeutically effective proteins.
In the case of the Covid-19 vaccine, these are the proteins of the characteristic spikes on the surface of the Corona virus which are used for vaccination. The proteins are presented on the surface of immune cells; then the human immune system triggers defenses against these foreign proteins and thus against the Corona virus. The mRNA itself is completely broken down after only a few hours, a fact which is advantageous to the safety of these vaccines.
The road to the best packaging
The mRNA has to be packaged appropriately in order to keep it from being broken down on the way to the cell by the ubiquitous enzymes of the human body. This is done using nanoparticles which can consist of a mixture of lipids or polymers.
The lipids are fat molecules similar to the molecules of the cell membrane and help deposit the mRNA in the interior of the cell. Lipids and biopolymers are then broken down or excreted by the body.
To this ends, the BioNTech formulation team led by Dr. Heinrich Haas worked together with the group led by Prof. Peter Langguth of the Pharmaceutical Technology department at the Johannes Gutenberg University Mainz's Institute of Pharmaceutical and Biomedical Sciences. They developed a series of formulations in which the nanoparticles consisted of various mixtures of lipids and biopolymers already proved in pharmaceuticals.
In the light of neutrons
In order to compare the properties of variously composed nanoparticles with one another, the researchers subjected the nanoparticles to a wide range of investigations. In addition to x-ray and microscopic analyses, these investigations included radiation with neutrons using the instrument KWS-2, operated by the Forschungszentrum Jülich at the FRM II of the Technical University of Munich in Garching.
The neutrons are scattered in the interior of the nanoparticles, inter alia, on the hydrogen nuclei and are deflected from their paths in a characteristic way. This is the basis for conclusions about their distribution. If the hydrogen atoms of certain components – for example of the lipids only – are exchanged with heavy hydrogen, the chemical properties and the pharmaceutical efficacy do not change, but the scattering pattern of the neutrons does.
"This method makes it possible to selectively highlight parts of a complex multi-component morphology without changing the physical chemistry of the sample," says Dr. Aurel Radulescu of the Jülich Centre for Neutron Science (JCNS), who is responsible for the instrument KWS-2 and who led the evaluation of the measurement results. "This makes it possible to depict structural properties which other methods can only barely render visible, if at all."
The right degree of order is the key
In these analyses the research teams were interested in how efficiently the various formulations were able to transmit the mRNA into the cell, referred to as transfection. The researchers thus found out that the highest transfection rates were achieved with nanoparticles that are characterized by a certain type of internal arrangement.
"High levels of biological activity were registered whenever ordered and less ordered areas alternated in the interior of the nanoparticles in a characteristic manner. This could be a generally valid concept of structure-activity relationship which can be applied independently of the systems investigated here," Dr. Heinrich Haas of BioNTech points out. A similarly low degree of order had also been found previously by the research teams using x-ray radiation in other lipid nanoparticles.
An improved procedure
In order to receive the desired structural properties lipids and biopolymers had to be combined with the mRNA using exactly defined procedures. Here the research team was able to show that the nanoparticles for packaging the mRNA could be produced in a single step, which means a significant simplification compared to the two-step procedure which was originally also investigated.
Thus a simplified method for the creation of mRNA nanoparticles with improved activity was ultimately found. "Such questions of practical producibility represent an important prerequisite for the possibility of developing pharmaceutical products," says Prof. Langguth. In the future such concepts could be taken into account in the development of new mRNA-based therapeutic agents.
Original publication
Christian D. Siewert, Heinrich Haas, Vera Cornet, Sara S. Nogueira, Thomas Nawroth, Lukas Uebbing, Antje Ziller, Jozef Al-Gousous, Aurel Radulescu, Martin A. Schroer, Clement E. Blanchet, Dmitri I. Svergun, Markus P. Radsak, Ugur Sahin and Peter Langguth; "Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for mRNA"; Cells; 2020, 9(9), 2034.
C. Siewert, H. Haas, T. Nawroth, A. Ziller, S. S. Nogueira, M. A. Schroer, C. E. Blanchet, D. I. Sverg, A. Radulescu, F. Bates, Y. Huesemann, M. P. Radsak, U. Sahin, P. Langguth; "Investigation of charge ratio variation in mRNA – DEAE-dextran polyplex delivery systems"; Biomaterials; 2019.
S S. Nogueira, A. Schlegel, K. Maxeiner, B. Weber, M. Barz, M. A. Schroer, C. E. Blanchet, D. I. Svergun, S. Ramishetti, D. Peer, P. Langguth, U. Sahin, H. Haas; "Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery"; ACS Appl. Nano Mater.; 2020, 3, 11, 10634–10645.