When spider venom attacks the nerves
Research team examines neurotoxin from a Black Widow
Although many people lose their nerve and panic when they see a spider, only very few of the creatures are actually dangerous. The Black Widow, however, is a force to be reckoned with: it catches its prey by means of nerve poison – to be precise, latrotoxins (LaTXs). Prof. Christos Gatsogiannis from the Institute of Medical Physics and Biophysics at Münster University investigated the substance – also with a view to medical applications. Using cryo-EM Gatsogiannis’ team succeeded in explaining the first structure of an LaTX.
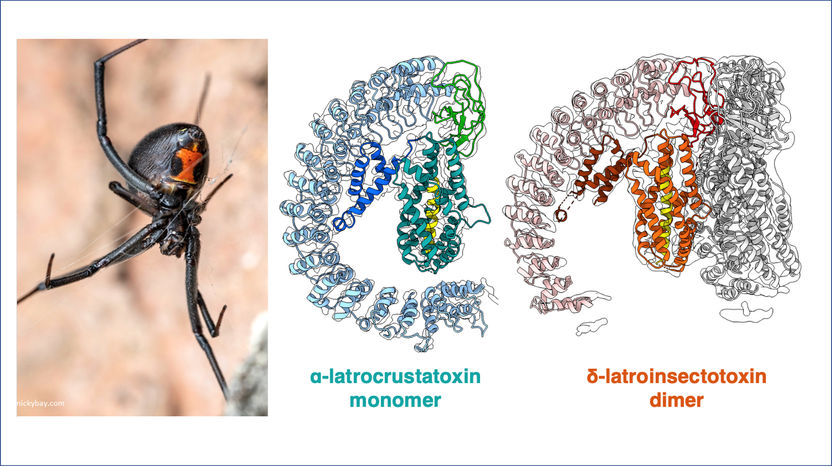
The team used cryo-electron microscopy to reveal the structures of toxins specific to insects and crustaceans (right) from the Black Widow (left).
copyright nickybay.com / AG Gatsogiannis
Phobias are often irrational by nature – especially in the case of spiders, as these creatures are usually more afraid of humans than vice-versa. But: some species are a force to be reckoned with – for example, the Latrodectus spider, more commonly known as the Black Widow. It catches its prey by using venom – to be precise, latrotoxins (LaTXs), a subclass of neurotoxins, or nerve poisons. A bite from a Black Widow can be fatal for humans. The exact structure of the nerve poison was previously unclear, but Prof. Christos Gatsogiannis from the Institute of Medical Physics and Biophysics at Münster University investigated the substance – not only because of its uniqueness, but also with a view to possible medical applications. Using cryo-EM, and in collaboration with Gatsogiannis’ former colleagues at the Max Planck Institute in Dortmund and with researchers at Jacobs University Bremen, the team of Münster researchers succeeded in explaining the first structure of a latrotoxin. The team’s findings have now been published in the Nature Communications journal.
Neurotoxins are probably known to many non-specialists – in the form of botox, which is often used in cosmetic surgery. The Black Widow’s poison, however, has anything but a “beautifying” effect: LaTX was developed by nature primarily in order to immobilise insects – or simply kill them straight off. In the process, the toxins dock onto specific receptors on the surface of nerve cells and cause neurotransmitters to be released, for example through a calcium channel. As a result of the constant inflow of calcium ions into the cell, transmitters are given off which lead to seizures.
This mechanism is what distinguishes the latrotoxins from all other variants of the so-called pore-forming toxins. “Despite wide-ranging studies carried out over many years, we didn’t know the structure of these toxins,” says Gatsogiannis. “For this reason weren’t able to understand the precise active mechanism.” Help was provided in the form of cryo-electron microscopy, or cryo-EM for short. By means of this three-dimensional method, biomolecules can now be “photographed” down to atomic resolution. In the process, the protein complexes in liquid ethane are frozen at minus 196 degrees, in milliseconds, into a thin layer of amorphous ice, a form of solid water. Hundreds and thousands of images are then captured which show different views of the proteins and, in this way, enable the structure of the neurotoxin to be recognised.
Using cryo-EM, and in collaboration with researchers at the Max Planck Institute in Dortmund and at Jacobs University Bremen, the team of Münster researchers succeeded in explaining the first structure of a latrotoxin. “The general structure of LaTX is unique and is different in every possible way from all other known toxins,” says Gatsogiannis. The new insights are fundamental for understanding the molecular mechanism of the LaTX family, and they pave the way for possible medical applications – as well as for the development of an efficient antidote. In addition, these insights into insect-specific toxins might open up new opportunities for pesticides. For future research, however, it is essential to understand how exactly the toxin is inserted into the membrane, i.e. into the surface of the cell. “At the moment we are studying the structure of all members of the latrotoxin family – in particular how they exactly recognise the specific receptors on the surface of the cell, and how these sensors function,” Gatsogiannis explains.
The biggest obstacle to these plans is the fact that cryo-EM is not yet available in the Münster area. Prof. Gatsogiannis and his team want to change this: “The practical importance for medical research is immense,” says Dr. Minghao Chen, the lead author of the study now published, “as ‘function’ is directly linked to ‘structure’ in a biological context. But the method is highly complex and requires an ultramodern infrastructure.” The research team plans to introduce this innovative method soon in Münster University’s new research building, the Center for Soft Nanoscience (SoN).
























































