How proteins clump together
Molecular mechanisms in the aggregation of proteins in membraneless organelles
What happens when cells are under prolonged stress? proteins behave atypically, stop working, can clump together and ultimately damage the entire cell. Researchers at Technische Universität Braunschweig and the Université Paris have investigated which processes lead to this. The focus was on a protein that is associated with familial ALS. If the molecular processes can be better understood, it could be possible to work on novel therapeutic approaches for neurodegenerative diseases. The results of the research work have been published in the Journal of the American Chemical Society.
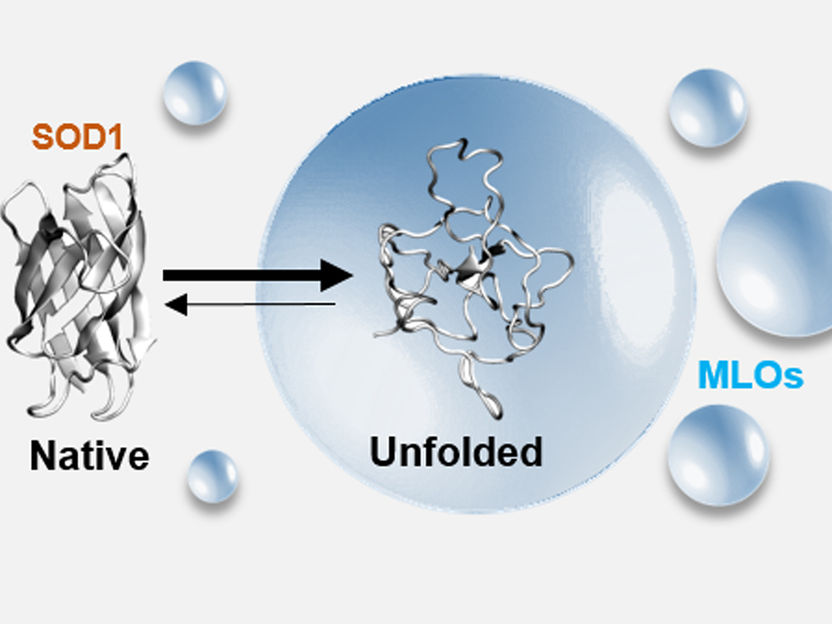
What happens when the cell is under stress: proteins (here: variant of the SOD1 protein) unfold and accumulate and clump together in so-called membraneless organelles (MLO).
Nirnay Samanta, Sara S. Ribeiro/TU Braunschweig
Proteins are the most important molecular machines of the cell, involved in all vital cellular processes. For a long time, cells have been regarded as “bags full of proteins”, with the cell components being subdivided by membrane-bounded organelles. Examples of these membrane-bounded organelles are nuclei and mitochondria, the “power plants of the cell”. However, research in the last five years has increasingly shown that proteins within such “bags” can additionally organize themselves into membrane-less organelles (MLOs).
The formation of these MLOs is based on liquid-liquid phase separation, i.e. it is comparable to the formation of oil droplets in water. This enables the cells to create different, separate chemical environments in which, for example, molecular processes can now take place very efficiently.
Some of these MLOs are formed to help the cell cope with stress. In this way, MLOs ensure that the functional form of the proteins is maintained, thus ensuring the survival of the cell. However, if the cell is exposed to severe stress over a long period of time, for example in the course of a disease, this mechanism may no longer function. Proteins clump together within the MLOs, can no longer perform their function or even damage the cell. These processes are associated with various neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease and Parkinson’s disease.
“The aim of our research is to understand the molecular mechanisms underlying the clumping of proteins in MLOs,” says Professor Simon Ebbinghaus from the Institute of Physical and Theoretical Chemistry at TU Braunschweig.
In the published research work, the protein superoxide dismutase 1 (SOD1) was investigated in particular. SOD1 is an enzyme that is associated with familial ALS. Various experiments and computer simulations were used to analyze the steps leading up to clumping. “We have shown that the initial clumping steps, the destabilization of individual SOD1 proteins, already play a crucial role. SOD1 proteins that have mutations associated with ALS accumulate thereby rapidly in MLOs. Through these novel findings, we hope to provide starting points for novel therapeutic strategies against serious neurodegenerative diseases. Our future research will continue in this direction,” says Professor Ebbinghaus.
Original publication
Most read news
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Provepharm signs license agreement with Daiichi Sankyo to provide methylthioninium chloride Proveblue solution for injection to Japan - Daiichi Sankyo will resolve the unmet need for an approved methylthioninium chloride injectable drug in Japan