Stem cells from the bioreactor
Process for the mass production
With the aid of artificial stem cells, it will soon be possible to establish new treatments for previously incurable diseases such as Alzheimer’s disease. At the Fraunhofer Project Center for Stem Cell Process Engineering SPT, a process for the mass production of these so called induced pluripotent stem cells is being developed. This process involves new materials, which ensure that industrial cell production will meet high quality standards. The process will be unveiled to the public for the first time at the MEDICA trade fair in Düsseldorf, Germany, between November 15 and 18, 2021.
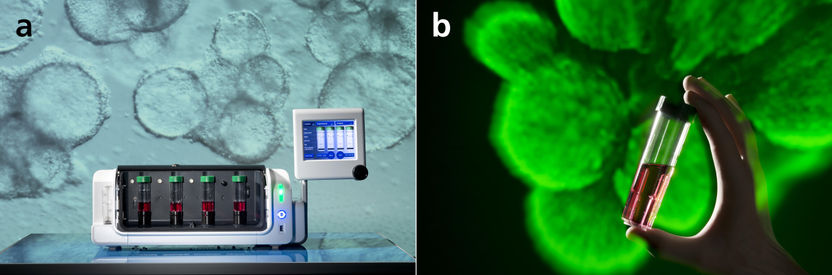
Scalable stem cell processing technology in suspension bioreactors (a). The different conditions are tested in separate cultivation tubes (b).
© Fraunhofer IBMT/Bernd Müller
The cells of a growing embryo have a fascinating property. They are able to transform into any other cell type – heart muscle cells, nerve cells and many more. Experts refer to them as pluripotent stem cells. When the human body is fully developed, the cells lose their pluripotency. Recently, however, it has become possible to artificially revert the somatic cells of an adult to their embryonic state. These “induced pluripotent stem cells” (iPS) are an important tool for biotechnologists. This means that somatic cells can be taken from adults with genetic diseases, reprogrammed into iPS cells and then differentiated into heart muscle or nerve cells, which can then be used to test new drugs. Because the iPS cells contain the patient’s genome, it’s much easier to determine which treatments will be effective for that patient. In this respect, iPS cells have huge potential for personalized medicine. The problem is that thus far, it hasn’t been possible to produce iPS cells and culture them in large quantities and to a high quality. There lacks a standardized production line and process for industrial manufacturing that meets regulatory requirements. The new Fraunhofer Project Center for Stem Cell Process Engineering SPT in Würzburg, which is jointly operated by the Fraunhofer Institute for Biomedical Engineering IBMT and the Fraunhofer Institute for Silicate Research ISC, is working on exactly that.
Combining materials expertise with biotechnology
“In this project, we’re combining our biotechnology expertise with the materials science know-how,” says the Managing Director of SPT, Julia Neubauer of the Fraunhofer IBMT. The Fraunhofer ISC is sharing its vast experience in the development of materials and surface coatings to help optimize the Fraunhofer IBMT biotechnologies for cell culture at the project center. Around the world, most scientists currently use hard, smooth surfaces for cell culture. On this type of surface, cells such as artificial heart muscle cells that actually contract can be grown. They do, however, behave differently to cells in a living body. For example, they hardly react to hormones such as adrenaline, which should make them beat faster. The SPT team is creating more realistic culture conditions so that the iPS cells will develop into more mature cells that behave naturally. Instead of hard surfaces, soft surface coatings made of hydrogels and 3D “biomimetic structures” made with 3D printers are used, for example. The surfaces are also modified with biochemical processes so that tissue-specific environment can be simulated.
The key to iPS cell culture at SPT is the use of bioreactors, in which the cells grow while floating freely in a nutrient solution or adherent on microcarrier. Julia Neubauer and Marco Metzger of the Fraunhofer ISC, the SPT's Deputy Managing Director, explain that in these reactors, the cells are cultured on tiny hydrogel microspheres, for example. They can also grow in small 3D aggregates and interact with each other. These methods successfully mimic natural tissue growth. Another advantage of free-floating iPS cell culture is that the cells receive optimal nutrition and oxygen. In a group of cells on a 2D surface, those on the bottom layer are often less well nourished. The cell environment also doesn’t provide natural conditions.
Cell biobank for research
Over the past few years, the Fraunhofer IBMT has worked with pharmaceutical companies to establish the European Bank For Induced Pluripotent Stem Cells (EBISC) – a unique biobank for iPS cells. This biobank provides a variety of different iPS cell lines – for example from patients with Parkinson's disease or early-onset Alzheimer's disease. The iPS cells are available to academia, companies and research institutes. “With the help of these iPS cells, treatments for a range of diseases can be developed and tested,” says Julia Neubauer. “The bank is thus of utmost importance for the future treatment of diseases that are currently incurable.”