Boehringer Ingelheim and Partners to Accelerate Development of First-In-Class Gene Therapy for Patients with Cystic Fibrosis
Boehringer Ingelheim, IP Group, the UK cystic fibrosis gene therapy Consortium (GTC, consisting of researchers from Imperial College London and the Universities of Oxford and Edinburgh) and Oxford Biomedica (OXB), announced today that Boehringer Ingelheim has exercised its options on intellectual property and know-how from the partners to progress and further accelerate the development of a potential, new treatment option for patients with CF. In the partnership, IP Group, acting on behalf of the three GTC host Universities, is granting exclusive global rights to develop, manufacture, register, and commercialize this lentiviral vector-based gene therapy for the treatment of cystic fibrosis. The GTC is additionally contributing its knowledge in pre-clinical research and clinical gene therapy development. OXB is adding its leading competence in manufacturing lentiviral vector-based therapies to Boehringer Ingelheim’s expertise in the development of novel breakthrough therapies for respiratory diseases.
oracast / Pixabay
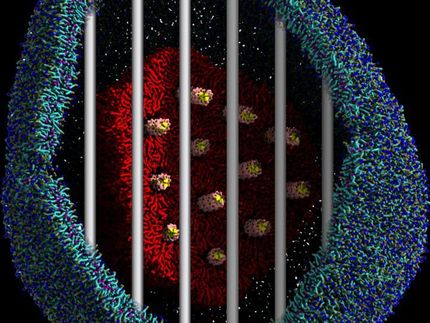
CF is a rare, progressive, life-threatening disease that results in severe dysfunction and persistent infections of the lung affecting 70,000 people worldwide. It is caused by a defective or absent protein that results from mutations in the CFTR gene. This innovative development partnership among academia, life science investors, pharma, and biotech focusses on the advancement of BI 3720931, a novel, replication deficient lentiviral vector, in an inhaled formulation, which selectively introduces a healthy CFTR gene into the relevant target cells.
Under the terms of the option and license agreement with Boehringer Ingelheim, originally announced in August 2018 , Boehringer Ingelheim will pay IP Group, on behalf of the GTC, an option exercise fee, near term, success based development, regulatory and sales milestone payments as well as royalties on net sales. OXB will receive an option exercise fee of £3.5 million and will be entitled to payments in an aggregate amount of up to £27.5 million upon achievement of various development, regulatory and sales milestones, in addition to a tiered low single digit royalty on net sales of a cystic fibrosis gene therapy product.
Other news from the department research and development
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.