Pfizer Starts Study of mRNA-Based Next Generation Flu Vaccine Program
The mRNA technology could to improve upon the efficacy of current flu vaccines
Pfizer Inc. announced that the first participants have been dosed in a Phase 1 clinical trial to evaluate the safety, tolerability, and immunogenicity of a single dose quadrivalent mRNA vaccine against influenza in healthy adults. Pfizer’s mRNA influenza vaccine program is the first in a planned wave of programs leveraging mRNA technology for influenza. Beyond influenza, the company plans to explore mRNA in other respiratory viruses, including medically appropriate vaccines combinations that could provide protection against more than one respiratory virus, as well as expand to develop mRNA technology in oncology, and genetic diseases.
Symbolic image
pixabay.com
“Since 2018, we have been working to develop a potential mRNA influenza vaccine, driven by our deep understanding of infectious diseases and our extensive experience in researching, developing and implementing new vaccine technologies to help prevent them,” said Kathrin U. Jansen, Ph.D., Senior Vice President and Head of Vaccine Research & Development at Pfizer. “The COVID-19 pandemic allowed us to deliver on the immense scientific opportunity of mRNA. Influenza remains an area where we see a need for vaccines which could result in improved efficacy in any given season, and we believe mRNA is the ideal technology to take on this challenge to transform global health outcomes.”
Conventional seasonal influenza vaccines are generally developed by growing the virus in chicken eggs or mammalian cells, which are inactivated and processed to be made into a vaccine. This process faces multiple challenges, including producing immunogenic antigens, keeping up with virus strain changes, and alterations in the vaccine antigens during production. With circulating influenza strains continually changing, predicting the best match for the next season’s vaccine is difficult for global health experts as those strains are chosen more than six months before the start of the influenza season that they target in the Northern Hemisphere.
Even when the vaccine strains match circulating influenza virus strains well, current seasonal vaccines typically confer 40% to 60% protection against circulating strains, with even lower protection in years with poor matching of strains. Influenza causes approximately 5 million cases of severe illness and up to 650,000 deaths worldwide every year.
mRNA-based influenza vaccine design requires only the genetic sequence of the virus. The flexibility of mRNA technology and its rapid manufacturing could potentially allow better strain match, greater reliability of supply, and the potential opportunity to improve upon the efficacy of current flu vaccines. Furthermore, in a pandemic influenza situation, mRNA technology could allow rapid, large-scale manufacturing of effective vaccines.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Asha_Choudhury
Patrick_Manson
Phialophora_gregata
Fog_fever
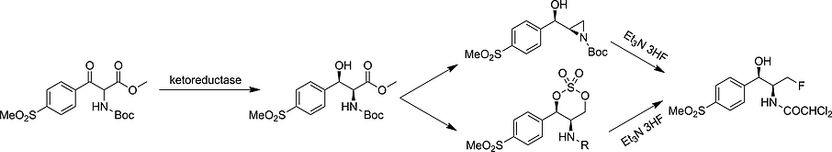
Two new ways to fluorinated structures - Chemo-enzymatic synthesis of fluorinated antibiotics

New technique could increase success rate, life span of implantable devices
Common blood test could predict risk of recurrent stroke

Light regulates structural conversion of chiral molecules - The conversion is relevant e.g. for the preparation of drugs