Bridging antibodies plus enhancer can destroy breast cancer cells
Scientists from the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) have developed antibodies that have two antigen-binding sites and can couple cancer cells with effector cells of the immune system. In laboratory tests, these bridging antibodies, together with an enhancer antibody, were able to specifically mobilize the body's own immune defenses and destroy breast cancer cells.
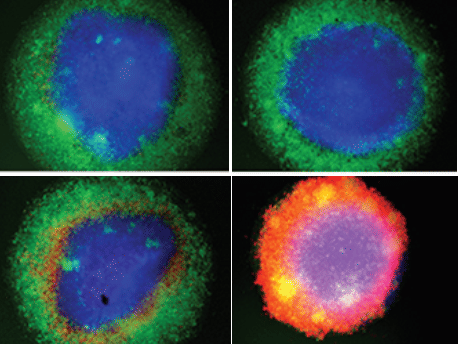
Fluroescence microscopy of breast cancer organoids: Cancer cells fluoresce blue, T cells gre only when bridging and enhancer antibodies interact at the organoid is significant activation of T cells observed. The T cells migrate into the organoid and the cancer cells largely die (red).
© Momburg/DKFZ
For some years now, bispecific antibodies (BiMAb: bispecific monoclonal antibodies) have enriched the spectrum of cancer immunotherapies. These are antibodies that have two different antigen binding sites. With one, they dock to a tumor-specific molecule on cancer cells. With the other, they attach to an antigen localized on the surface of immune defense cells. The bridging function of the bispecific antibodies brings the cancer cell and the immune cell into physical contact with each other, allowing the immune system to target the tumor and, ideally, ultimately destroy it.
In the fight against blood cancer, BiMAb have already proven to be highly effective in certain disease situations. Experiments to combat solid tumors such as breast cancer, melanoma ans sarcoma, on the other hand, have so far been less successful.
Researchers at the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ), led by Frank Momburg, together with a research group led by Dirk Jäger, National Center for Tumor Diseases (NCT) Heidelberg, have now succeeded in developing BiMAb that specifically activate the immune defense in the cell culture dish. The scientists were even able to achieve this effect on millimeter-sized spherical cell clusters of breast cancer cells, known as tumor organoids.
To this end, they constructed a series of antibody variants, each of which was directed against an antigen characteristic of breast cancer and also against the T-cell molecule CD3. Binding of the antibodies stimulated T cells to secrete messenger molecules (cytokines) and activated cell-killing mechanisms. "The challenge here is to produce bispecific antibodies against tumor antigens and CD3 that are so selective that they act exclusively on tumor tissue but do not attack surrounding healthy tissue," Momburg explains. "This is not an easy task, since many tumor antigens are also present to a small extent in healthy tissue."
Another challenge is that, unlike blood cancer cells, cells of solid tumors are physically more difficult for antibodies to access. In addition, the environment surrounding tumors is usually immunosuppressive, which further impairs defense mechanisms. To address these challenges and amplify the T cell response at the tumor, Karsten Warwas and colleagues used additional bispecific antibodies that establish contact between additional pairs of molecules on the tumor and T cell. These enhancer antibodies do not activate the T cells directly, but amplify the signal of the activating BiMAbs by "co-stimulation", which can overcome immune suppression. This approach has allowed the dose of T cell-activating BiMAb to be reduced to a minimum. This may help in a therapeutic situation to avoid toxic side effects of antibodies due to excessive release of cytokines.
The laboratory experiments with breast cancer organoids showed that the combination of bridging and enhancer antibodies was able to specifically mobilize T cells, and only in samples that also contained cancer cells carrying the tumor antigen. "Thus, T-cell activation occurred only when both cell types - tumor cells and T cells - encountered each other in close proximity. With a view to later application in humans, this is a very crucial safety aspect," Momburg said.
The new method may one day find application in cancer immunotherapy, but must now first be tested further before it finds its way into clinical trials. Frank Momburg explains: "In our approach, stimulatory and costimulatory BiMAb complement each other. This principle could be beneficial for the treatment of solid tumors with sparsely expressed tumor antigens."
Original publication
Karsten M. Warwas, Marten Meyer, Márcia Goncalves, Gerhard Moldenhauer, Nasja Bulbuc, Susanne Knabe, Claudia Luckner-Minden; Claudia Ziegelmeier, Claus Peter Heussel, Inka Zörnig, Dirk Jäger und Frank Momburg; "Co-Stimulatory Bispecific Antibodies Induce Enhanced T Cell Activation and Tumor Cell Killing in Breast Cancer Models"; Frontiers in Immunology; 2021
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Antibodies
Antibodies are specialized molecules of our immune system that can specifically recognize and neutralize pathogens or foreign substances. Antibody research in biotech and pharma has recognized this natural defense potential and is working intensively to make it therapeutically useful. From monoclonal antibodies used against cancer or autoimmune diseases to antibody-drug conjugates that specifically transport drugs to disease cells - the possibilities are enormous

Topic world Antibodies
Antibodies are specialized molecules of our immune system that can specifically recognize and neutralize pathogens or foreign substances. Antibody research in biotech and pharma has recognized this natural defense potential and is working intensively to make it therapeutically useful. From monoclonal antibodies used against cancer or autoimmune diseases to antibody-drug conjugates that specifically transport drugs to disease cells - the possibilities are enormous




















































