Programming synthetic exosomes to optimize wound healing
Scientists create synthetic exosomes with natural functionalities and present their therapeutic application
Scientists from the MPI for Medical Research and colleagues have engineered synthetic exosomes that regulate cellular signaling during wound closure. The synthetic structures resemble naturally occurring extracellular vesicles (EV) that play a fundamental role in communication between cells. The scientist uncovered key mechanisms to regulate and aid wound healing and the formation of new blood vessels. Inspired by the function of their natural blue prints, the scientists successfully demonstrate for the first time that fully-synthetic exosomes with therapeutic functionality can be constructed. The results were recently published in Science Advances.
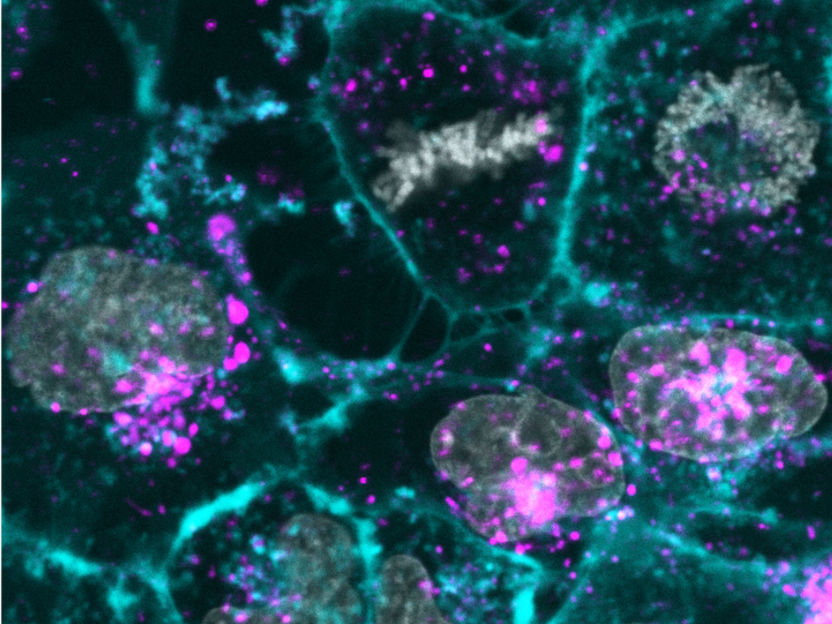
Human skin cells cultures (gray and cyan) are reprogrammed by the uptake of synthetic exosomes (purple).
MPI for Medical Research
Extracellular vesicles mediate communication between cells
A well-working communication between cells is fundamental for multicellular organism such as ourselves. Almost all processes in our body require a coordinated interaction between cells as they form tissues and organs or, for example, work together during immune responses. Wound healing or the formation of new blood vessels also requires extensive cell-cell-signaling to ensure coordinated tissue regeneration. For this, cells in the skin apply several mechanisms to communicate with each other. One of those are extracellular vesicles (EVs). They are small droplets or compartments that cells can load with different molecules and release to transfer “information”. Upon binding to other cells or uptake by those, they release that information. However, little is known about the concrete mechanisms and the complex signaling processes working behind extracellular vesicle communication. This is also the major limiting factor for their therapeutic application.
Learning-by-building: fully-synthetic extracellular vesicles tweak the system
To develop a better understanding of EVs, scientists at the MPIMR and collaborators chose a bottom-up synthetic biology approach. Within test tubes, they designed and assembled fully-synthetic extracellular vesicles to precisely mimic their natural role model in form and function. By this, they aimed for a better, more systematic assessment of their role during wound healing and to understand the relevance and function of every single component within the vesicles. Their bottom-up approach, using lipid droplets, enabled to fine-tune the functionalities of the EVs and create a programmable EV technology. “We can potentially create synthetic vesicles with various kinds of characteristics – that is the beauty of the bottom-up approach. It gives us the chance to copy, tune and improve natural structures for different purposes”, says Oskar Staufer, first author and postdoctoral researcher in the Cellular Biophysics Department at the MPIMR.
Therapeutic use of synthetic EVs during wound healing
To demonstrate the functionality of the synthetic EVs during wound healing processes, the researchers studied the healing behavior of human donor skin cultured in the lab. When they treated wounds in these skins with their synthetic exosomes, they could show a much faster healing and the wounds closed faster. A similar observation was made when testing the synthetic exosomes´ ability to improve the formation of new blood vessels, another process that is extremely relevant in several therapeutic contexts such as tissue regeneration after surgery and cardiac damage. By the digging deeper into the mechanism that act within the cells treated with the synthetic EVs, the scientists were also able to determine crucial molecules within the EVs that are responsible for triggering the therapeutic effects. Identifying the key components of the EVs is a key advance that in future might enable engineering individually adjustable vesicles of therapeutic value for the treatment of many diseases like cancer, immune-disorders or neurodegenerative diseases.
“Exosomes have raised high hopes in biomedicine for more than 20 years now. With this new technology in hands, we are now able to synthetize exosomes with high purity on a therapeutic scale. This overcomes some of the major limitations that have impeded the application of exosomes for therapy and could lead us to harness the power of extracellular vesicle communication. Moreover, as we are working with very defined vesicle systems, we are now able to study the fundamental functions of EV in a very systematic way.”