Using yeast to create alternative petrochemical processes
Integrating cellular engineering with cell-free biosynthesis could lead to efficient ways to power the earth
As climate change continues to do more damage to our planet, scientists are working to find more efficient and cleaner ways to power the earth. One appealing alternative to common petrochemical processes that generate significant greenhouse gases and other waste products could come from biological systems.

Symbolic image
pixabay.com
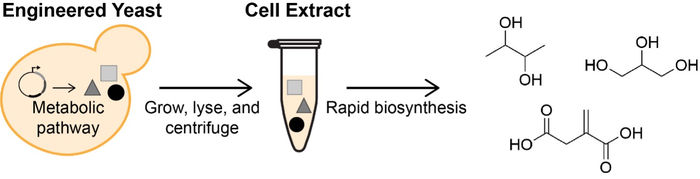
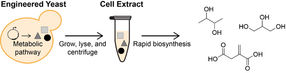
Yeast strains engineered for the biochemical conversion of glucose to value-added products are limited in chemical output due to growth and viability constraints. Cell extracts provide an alternative format for chemical synthesis in the absence of cell growth by isolating the soluble components of lysed cells. By separating the production of enzymes (during growth) and the biochemical production process (in cell-free reactions), this framework enables biosynthesis of diverse chemical products at volumetric productivities greater than the source strains.
Blake Rasor

Recent work from Northwestern Engineering’s Michael Jewett and researchers from the University of Texas at Austin has led to advances in understanding of biochemical pathways and increased rates of chemical production by biological systems. The findings could bring us closer to implementing sustainable alternatives to synthesizing materials, fuels, and other oil-derived products.
The paper “An Integrated In Vivo/In Vitro Framework to Enhance Cell-Free Biosynthesis with Metabolically Rewired Yeast Extracts,” published Aug. 26 in the journal Nature Communications, describes the development of optimized in vitro biosynthesis (biochemical production) processes that use cell extracts from engineered strains of Saccharomyces cerevisiae (brewer’s yeast). Along with Jewett, Walter P. Murphy Professor of Chemical and Biological Engineering at the McCormick School of Engineering, the paper was written by Blake Rasor, a PhD student in Jewett’s lab. The investigation was conducted in collaboration with the research group of Hal Alper, a professor at the University of Texas at Austin.
The work was supported through the US Department of Energy Joint Genome Institute’s Emerging Technologies Opportunity Program (ETOP). The ETOP provides funding to seed the development of new technologies offered to those researchers worldwide who tap JGI’s user programs to advance energy and environmental applications.
Decades of metabolic studies and genetic tool development make S. cerevisiae a highly controllable framework for biochemical production. Beyond historical applications in baking and brewing, this yeast has been engineered to produce innumerable target molecules used in industrial and therapeutic applications.
However, cellular production systems have an internal tug-of-war between making more cells and making the engineered product. Jewett’s group avoids these growth and viability constraints by breaking the biological machinery out of cells and using the extracted material for cell-free biochemical reactions, which enables the optimization of levers that are not easily tuned in living cells.
Previously, cell-free biosynthesis efforts with crude cell extracts have primarily used unmodified strains of E. coli. The researchers expanded the scope of this technique by using extracts from S. cerevisiae and by incorporating cellular metabolic engineering techniques to enhance the biosynthetic potential of cell-free reactions. This demonstrates that metabolic rewiring in cells produces extracts with greater volumetric outputs than wildtype (unchanged) extracts and the corresponding cell cultures.
Specifically, the cell-free production of three chemical products (butanediol, glycerol, itaconic acid) at a rate up to 10 times faster than corresponding cellular approaches points to the flexibility and efficacy of integrating cellular engineering with cell-free biosynthesis.
“This could expand the breadth of biological platforms underpinning efforts in sustainability,” Rasor said.
“Our work further joins an emerging area of science that seeks to use cell-free systems from crude cell extracts for designing cellular function, on-demand biomanufacturing, and portable diagnostics,” said Jewett, director of the Center for Synthetic Biology. “Indeed, these efforts are expanding the definition of biomanufacturing to build a sustainable bioeconomy.”
As for next steps, Jewett said he and his collaborators are building on this work both for pathway prototyping in the context of altered metabolism and for cell-free biomanufacturing to complement current cell-based approaches.
“Expanding the integrated cell/cell-free metabolic engineering strategy to yeast strains producing other value-added biochemical products and increasing the scale of cell-free reactions could spearhead the development of sustainable, economically viable alternatives to current chemical production processes,” he said.