Insulin can directly influence the internal clock in fat tissue
Eating at the wrong time of day disrupts our circadian rhythm
Advertisement
Eating at the wrong time of day disturbs our circadian rhythm and increases the risk of obesity and type 2 diabetes. The team led by PD Dr. Olga Ramich from DIfE, Professor Achim Kramer and Professor Andreas F. H. Pfeiffer from Charité - Universitätsmedizin Berlin provides the first explanations for this phenomenon in a new study. The researchers have shown for the first time in human samples that insulin can influence the internal clock of adipose tissue.

"Our results show for the first time the way in which inappropriate eating times can disrupt our circadian rhythms and cause negative metabolic changes," Ramich concludes (symbolic image).
pixabay.com
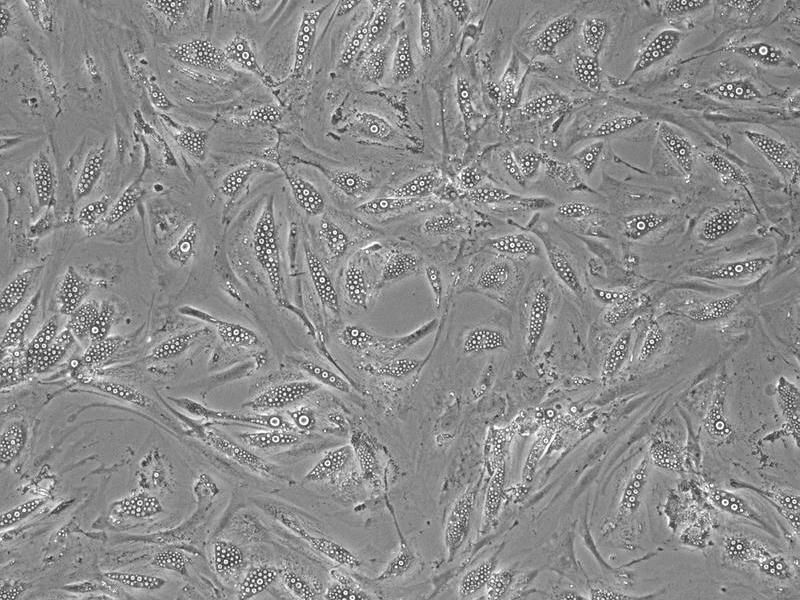
Microscopic image of human adipocytes in which lipid droplets (white) accumulate.
DIfE

Clocked from head to toe
Our internal clock controls almost all physiological processes, for example metabolism, blood pressure and body temperature. In addition to the central internal clock, located in the suprachiasmatic nucleus of the hypothalamus, there are many subordinate clocks found in every organ, tissue and cell of the body. Circadian rhythms are based on a tight interplay of so-called clock genes that generate a 24-hour rhythm via interlocking feedback loops. Recent studies show that meal times and food composition can alter the circadian rhythms of various tissues. Metabolically active, insulin-sensitive tissues, such as liver and adipose tissue, are particularly affected. In addition, mealtimes that are out of sync with the body clock increase the risk of obesity and metabolic diseases such as metabolic syndrome and type 2 diabetes. However, little is known about the underlying mechanisms.
Insulin alters gene expression
For the current study, the research team led by Ramich, Kramer and Pfeiffer investigated the influence of increased insulin levels after a meal on the circadian rhythm of adipose tissue and which molecular mechanisms play a role in this. To do this, they analyzed adipose tissue samples from 17 obese, non-diabetic men taken before and four hours after the so-called hyperinsulinemic-euglycemic clamp. "This method is usually used to determine insulin sensitivity. But it was also ideal for our research question because it allowed us to study the pure effects of insulin on adipose tissue in humans in vivo," explains Ramich, who heads the Molecular Nutritional Medicine research group at DIfE. The scientists isolated genetic material from the adipose tissue samples and determined the activity of various genes. Compared to the control group, which had received saline instead of insulin, the researchers found a significantly altered expression of clock genes, which suggests an insulin-dependent regulation of the internal clock.
Circadian rhythms made visible
To elucidate the molecular mechanisms responsible for this regulation, the researchers used human and animal fat cells that had been genetically transformed in culture or isolated from a genetically modified mouse model. A luciferase gene was inserted into these cells and linked to a section of the Per2 gene. Per2 is one of the key genes of the molecular clockwork. Thanks to luciferase, the cells produce light in response to Per2 activity, which allowed the scientists to observe the circadian rhythms of Per2 in real time over several days. "We found that insulin causes a rapid and transient increase in Per2 activity, altering the overall clock rhythm," explains Dr. Neta Tuvia, who shares first authorship of the study with Dr. Olga Ramich.
Crucial gene segments identified
In molecular biological experiments, the researchers then identified those sections of the Per2 gene that are crucial for the insulin effect. Piece by piece, they trimmed the promoter - the DNA section that controls the expression of a gene - and found that the region between 64 and 43 base pairs plays an essential role. "Our results show for the first time the way in which inappropriate eating times can disrupt our circadian rhythms and cause negative metabolic changes," Ramich concludes. "This may also explain why eating at night has a particularly unfavorable effect on metabolism." The researchers assume that the mechanisms leading to the eating-induced change in the internal clock are even more complex and that further hormones and metabolites are involved. This will have to be investigated in future studies.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Tuvia, N., Pivovarova-Ramich, O., Murahovschi, V., Lück, S., Grudziecki, A., Ost, A. C., Kruse, M., Nikiforova, V. J., Osterhoff, M., Gottmann, P., Gögebakan, Ö., Sticht, C., Gretz, N., Schupp, M., Schürmann, A., Rudovich, N., Pfeiffer, A. F. H., Kramer, A.: Insulin directly regulates the circadian clock in adipose tissue. Diabetes (2021).























































