First synthetic tissue model developed in which blood vessels can grow
A research team headed by biomedical engineer Dr Britta Trappmann from the Max Planck Institute for Molecular biomedicine in Münster, Germany, has developed a cell culture system in which, for the first time, a functional blood vessel system is able to grow within a framework made of synthetic material. The team investigated which material properties promote individual parameters of vessel formation – a step towards the futuristic vision of implantable artificial tissues. The study has been published in the journal “Nature Communications”.
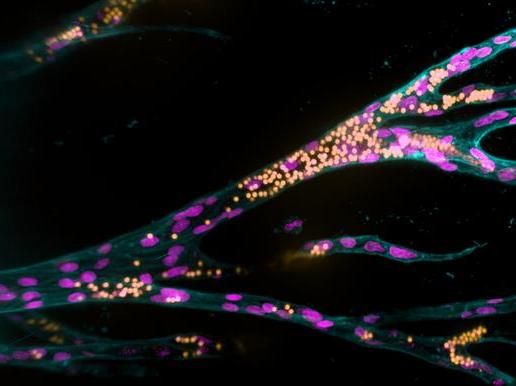
Growing from a parent blood vessel (upright on the left), endothelial cells (pink nuclei) form new blood vessels in a synthetic hydrogel. The fluorescent beads (yellow) simulate blood flow.
Jifeng Liu, MPI Münster
Using lab-created tissue to heal or replace damaged organs is one of the great visions for the future of medicine. Synthetic materials could be suitable as scaffolding for tissue because, unlike natural tissues, they remain stable in the organism long enough for the body to form new natural structures. A fundamental requirement for functional tissue is that blood vessels must be able to grow in them and connect to the organism’s vascular system, so that the tissue is properly supplied with oxygen and nutrients. However, until now, almost nothing has been known about which material properties promote the growth of blood vessels.
A team headed by biomedical engineer Dr Britta Trappmann from the Max Planck Institute for Molecular Biomedicine in Münster, Germany, has developed a cell culture system in which, for the first time, a functional blood vessel system is able to grow within a framework made of synthetic materials. The scientists, working in a special hydrogel with properties they can change in a controlled manner, first grew a parent blood vessel from human blood vessel lining cells. They then investigated how the material properties of the artificial cell environment influenced the formation of additional blood vessels and fine-tuned them. Summarizing the key findings, Britta Trappmann highlights that “The synthetic tissue material must activate certain adhesion molecules in the membrane of blood vessel cells so that the cells migrate in groups from the parent vessel and form tubular structures. At the same time, the material must be sufficiently degradable for the cells to form blood vessels of adequate size”. In order to mimic the natural environment of cells, many additional biomolecules and cells would have to be integrated into the model system in later steps – these may be signaling proteins, immune cells or cells to stabilize the blood vessels. “Moreover, the effect of all these factors is linked in natural tissues and varies from organ to organ,” Britta Trappmann explains. Understanding all of this, she says, is a long-term goal but, ultimately, the knowledge might then be used to grow implantable tissues.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Cell culture technology
Cell culture technology is a central pillar in biotechnological and pharmaceutical research and development. It enables the growth and maintenance of cells under controlled laboratory conditions, providing a window into the molecular and cellular processes of life.

Topic world Cell culture technology
Cell culture technology is a central pillar in biotechnological and pharmaceutical research and development. It enables the growth and maintenance of cells under controlled laboratory conditions, providing a window into the molecular and cellular processes of life.