In slow motion against antibiotic resistance
How novel therapeutics provide insight into bacteria membranes
Whether bacteria are resistant to antibiotics is often decided at the cell membrane. This is where antibiotics can be blocked on their way into the cell interior or catapulted from the inside to the outside. Macrocyclic peptides, a novel class of antibiotics, bioactive cytotoxins and inhibitors, shed light on how this transport process occurs at the membrane, how it is influenced and how it can be used to circumvent the resistance of a malignantly transformed cell. The research results, which were developed under the direction of Professor Robert Tampé (Goethe University) and Professor Hiroaki Suga (University of Tokyo), have been published in the journal eLife.
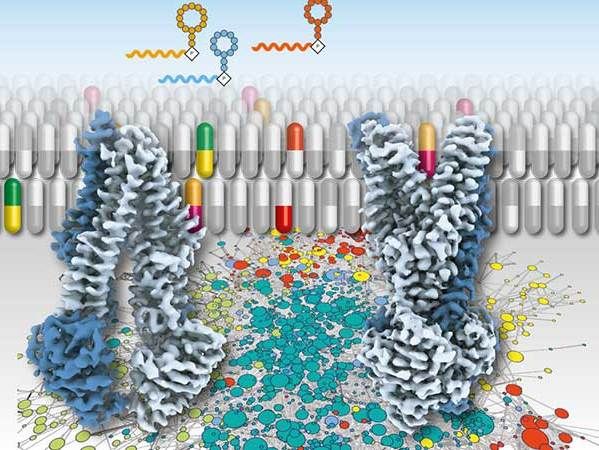
Synthetic therapeutics for antibiotic resistance in bacteria or multidrug resistance in tumour cells can block ATP-driven transport proteins that carries chemotherapeutics out of the cell.
Robert Tampé, Institute for Biochemistry, Biocentre, Goethe University Frankfurt
There are currently only a few synthetic agents that bind to and block the widespread membrane transport proteins, ATP-binding cassette transporters (ABC). Scientists at Goethe University and the University of Tokyo identified four of these macrocyclic peptides as models for a novel generation of active substances. They used methods for which the scientists involved are considered world leaders.
Thanks to deep sequencing, an extremely fast and efficient read-out procedure, the desired macrocyclic peptides could be filtered out of a "library" of macrocyclic peptides comprising trillions of variants (1 with 12 zeroes) - a number that exceeds the number of stars in the Milky Way. The fact that such an enormous amount exists at all is related to a novel procedure: By reprogramming the genetic code, amino acids can be used specifically as active components that are not otherwise used in the cell. In particular, their circular, closed structure distinguishes them from natural proteins. "Because these therapeutics are cyclic, they break down less rapidly in the cell," explains Robert Tampé, Director of the Institute of Biochemistry at Goethe University. "In addition, the ring-shaped active substances are restricted in their spatial structure, so they bind to the target molecule without major rearrangements." A third distinguishing feature makes macrocyclic peptides particularly attractive for scientists: When the active substances are produced, their building instructions are supplied as a "barcode". If certain therapeutics are selected from among a trillion synthetically produced ones, they carry their "name tags" with them, so to speak.
So what role do synthetic therapeutics play in antibiotic resistance in bacteria or multidrug resistance in tumour cells? What happens when they encounter the ATP-driven transport molecule that is responsible for resistance by carrying the chemotherapeutic agents out of the cell? In a nutshell: The drugs block the transporter by binding to it. This can happen at the beginning or at the end of a transport process, when the transporter is in a resting state. However, since the scientists can slow down the transport process so that it is carried out in slow motion, they can identify the agents that "enter" in the middle of the transport process and "hold" the membrane protein in its respective position. In this way, the researchers gain an insight into the choreography of the transport process as if through the images of a film strip.
These insights have already led to a "paradigm shift" in science, as Tampé explains: "Until now, we have assumed that ATP hydrolysis (note: an energy-releasing splitting process) provides the energy for transport through the membrane. However, this is only indirectly the case. It is the event of the binding of the ATP molecule that pushes substances out of the cell. The energy of hydrolysis, on the other hand, is used to return the ABC transporter to its initial state." The research groups at Goethe University and the University of Tokyo are convinced that these and other insights into membrane processes will point to the development of future medicines.
Basic research on cellular membranes and membrane proteins already has a long tradition in Frankfurt. Robert Tampé elucidated essential mechanisms of ATP-driven transport proteins and cellular machinery of adaptive immune response and quality control, which together with this new publication can provide approaches for applied drug research. Tampé was head of the Collaborative Research Centre "Transport and Communication across Biological Membranes" (SFB 807) which expired at the end of 2020. Meanwhile the concept for a new research centre on highly dynamic processes related to protein networks and machineries in cellular membranes is already under development. In the long term, the research results should reveal new possibilities for the therapy of molecular diseases, infections and cancer.