Engineering T cells for cancer therapy efficiently and safely
Innovative non-viral vector developed
Genetically enhancing a patient's immune cells by adding therapeutic genes to them outside the body is regarded as a promising new treatment approach in oncology. However, the production of these therapeutic cells using viruses is not only expensive but time-consuming. Researchers at the German Cancer Research Center (DKFZ) have developed an innovative non-viral vector that can efficiently introduce therapeutic genes into immune cells. At the National Center of Tumor Diseases (NCT) Heidelberg, therapeutic T cells produced with the novel vector were able to target and fight cancer more efficiently than conventionally produced cellular therapies.
Harbottle/DKFZ
How can the body fight tumors if they are invisible to the immune system? One solution to this problem involves the already established Chimeric Antigen Receptor (CAR) T cell therapy, in which a patient's T cells are modified outside the body by adding the genes for a synthetic receptor protein. This receptor is designed to recognize and attach to specific surface molecules on tumor cells. The genetically modified T cells are subsequently reintroduced into the patient where they can specifically target tumor cells.
CAR-T cell therapies have already been shown to be extremely successful in some types of blood cancer – with response rates of over 80 percent – and several CAR-T cell products have already been approved for clinical use. "A number of other areas of application are conceivable for CAR-T cells, but until now, it has been very expensive and time-consuming to produce them," Patrick Schmidt from NCT Heidelberg explained.
Typically, the genes for the synthetic receptor are inserted into T cells using viruses as genetic shuttles. This can have disadvantages, however: the retroviruses which are used integrate into the T cell DNA, and this could potentially lead to dangerous mutations. Transferring the genes as "naked" DNA – known as plasmids – has not been a viable option so far, as T cells react extremely sensitively to foreign DNA molecules.
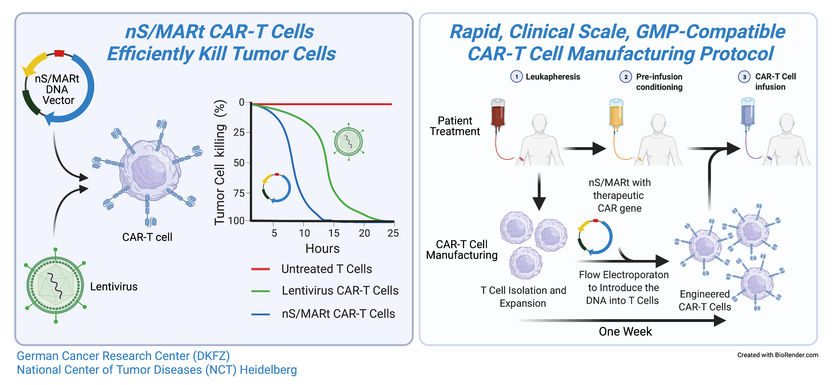
After many years of development, Matthias Bozza and Richard Harbottle from the DKFZ have now managed to construct a completely novel and patent-protected plasmid vector that is accepted by the T cells: This DNA construct does not integrate into the T cell's DNA, but instead replicates autonomously in the cell nucleus and is thus transferred to the daughter cells during T cell division.
The researchers use a particular region of human DNA to attach the vector to the matrix proteins of the cell's nucleus. They systematically refined this DNA construct, known as a nano-S/MARt DNA vector, to remove all the components of the vector that could provoke an immune reaction in the T cells. In addition, they also enhanced the efficiency with which the introduced gene is read in T cells. "By optimizing every component of the vector, we created a plasmid that is invisible to cells, and therefore it can be used to efficiently transfer genetic material into human T cells," explained Matthias Bozza, the first author of this study.
The nano-S/MARt vector construct can be transferred to T cells using a rapid and simple method known as electroporation. Using a semi-automated production system, the Heidelberg-based researchers managed to generate a sufficient amount of CAR-T cells, within the space of only five days, that could be used to treat patients. "That is a considerable improvement over the virus-based method, which typically takes about three weeks," explained Patrick Schmidt, who tested the technology as one of the two co-directors of the study.
The CAR-T cells produced using the nano-S/MARt vector proved to be more efficient at killing cancer cells than comparable cells generated using viruses. This was true both in experiments with tumor cells in a petri dish and in mice bearing tumors.
"There is great potential for using CAR-T cells and other cellular immunotherapies in oncology. The possibility of using a safer vector to manufacture large amounts of these kinds of therapeutic cells more rapidly and affordably marks a crucial step forward in harnessing the potential of this innovative form of treatment more effectively in the future," study co-director Richard Harbottle remarked. "The advantages of the nano-S/MARt vector system are so convincing that we can assume that our method sets a new standard in the production of genetically modified immune cells."
Original publication
Matthias Bozza, Alice De Roia, Margareta P. Correia, Aileen Berger, Alexandra Tuch, Andreas Schmidt, Inka Zörnig, Dirk Jäger, Patrick Schmidt & Richard P Harbottle; "A non-viral, non-integrating DNA Nanovector platform for the safe, rapid, and persistent manufacture of recombinant T Cells"; Science Advances; 2021