Putting pathogens on mute
Researchers develop novel strategy for treating hospital germs
Advertisement
The bacterium Pseudomonas aeruginosa is among the most common causes of pneumonia and poses a major challenge to hospitals worldwide. The treatment of infections with Pseudomonas aeruginosa is usually challenging because the bacterium forms so-called biofilms, which protect it from drugs. Dr Martin Empting and his team, in collaboration with several groups at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), have now developed an innovative method to disrupt the formation of these biofilms and thus facilitate the treatment of infections with Pseudomonas aeruginosa.
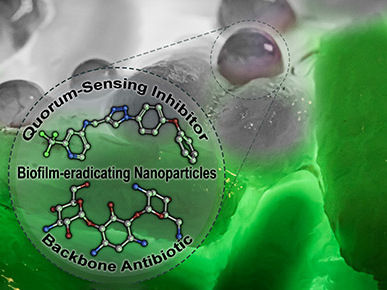
Dual-loaded nanoparticles carrying a quorum sensing inhibitor (QSI) and standard-of-care antibiotic tobramycin eradicate Pseudomonas aeruginosa biofilms. The QSI specifically disrupts the regulation of pathogenicity-determining traits and thereby potentiates antibiotic efficiency against this otherwise recalcitrant bacterium.
© Martin Empting/HIPS
Pseudomonas aeruginosa is capable of colonizing almost every part of the human body, making it the causative agent of a wide range of infectious diseases. The reason for the success of the bacterium lies, among other things, in its ability to produce and settle in biofilms. These biofilms consist of a mixture of different biomolecules including sugars, proteins, and lipids and provide the pathogen with protection against the human immune system as well as antibiotics. In addition, resistance to three or more of the commonly used classes of antibiotics occurs in about ten percent of all Pseudomonas infections in the EU, further exacerbating the situation.
To address the resulting need for innovative strategies and treatment options, researchers at HIPS, a site of the Helmholtz Centre for Infection Research (HZI) in collaboration with Saarland University, have now developed a promising strategy. Instead of directly killing the disease-causing bacteria, they are merely "disarmed”. In this specific case, it means that communication between the individual bacteria is blocked. Martin Empting, head of the study at HIPS, says: "Pseudomonas aeruginosa only forms biofilms and big amounts of pathogenic molecules when a sufficient number of bacteria come together. To sense when this point is reached, the pathogens need to communicate with each other. Our newly developed compounds target the protein PqsR, a key component of Pseudomonas aeruginosa's communication system, thereby disrupting the formation of both the biofilm and some bacterial toxins."
If the bacteria can no longer produce a biofilm, they are more accessible - both to cells of the human immune system and to conventional antibiotics. Martin Empting's team was able to demonstrate the latter in their study, which has now been published in the journal Advanced Science. When the novel PqsR inhibitors are administered together with the antibiotic tobramycin in a tailor-made nanoparticle formulation, the dose of antibiotic required to eradicate the biofilm decreases more than 30-fold. "At the end of the day, a drug is much more than just the active ingredient," says Prof Claus-Michael Lehr, head of the department Drug Transport across Biological Barriers. "This substance must reach its site of action in a targeted way and in high concentrations – otherwise it may promote adverse effects and, above all, the development of resistance. To this end, various biological barriers must be overcome, such as in this case the lungs, the biofilm formed by the bacteria, but also the bacterial cell wall. This is where nanocarriers can make a significant contribution."
In addition to the potent activity of the developed molecules, the researchers also put a special focus on their tolerability. "An important part of our work is to identify potential side effects as early as possible and avoid them by targeted chemical modification of the developed substances," says Prof Anna Hirsch, head of the Drug Design and Optimization department at HIPS. "Good efficacy is only half the story. Since we want to bring our molecules into clinical application, we must always keep an eye on how the substances behave in the body and optimize them as much as possible. The location in the body where the substances are needed also plays a role here." Based on the recent results, the new compounds show high potential for application in lung infections.
Prof Rolf Müller, Managing Director of HIPS and Head of the Department Microbial Natural Products, also sees high potential in the developed substances: "These new molecules are an excellent and innovative approach in the fight against resistant pathogens and infectious diseases in general. Since they do not kill pathogens directly, there is also significantly less selection pressure. This gives us reason to hope that resistance will only develop much more slowly as compared to antibiotics."
The potential of this type of "disarming" substance, also referred to as a pathoblocker, has also been recognized by the U.S. funding organization CARB-X, who has been funding Anna Hirsch's work in this field since late 2020 with a grant totaling 1.46 million euros.























































