Cells talk at each other to specialize different functions
New concept to describe how cells specialize during development
During development, cells must specialize their function in a well defined timeline: formation of different tissues must be coordinated from a pile of cells. The research group led by Aneta Koseska (former Max Planck Institute of Molecular Physiology (MPI), CAESAR Bonn) has now developed a new theoretical concept that shows how cells specialize in right proportions in a coordinated manner through their communication with each other, and thus how new structures are formed and maintained.
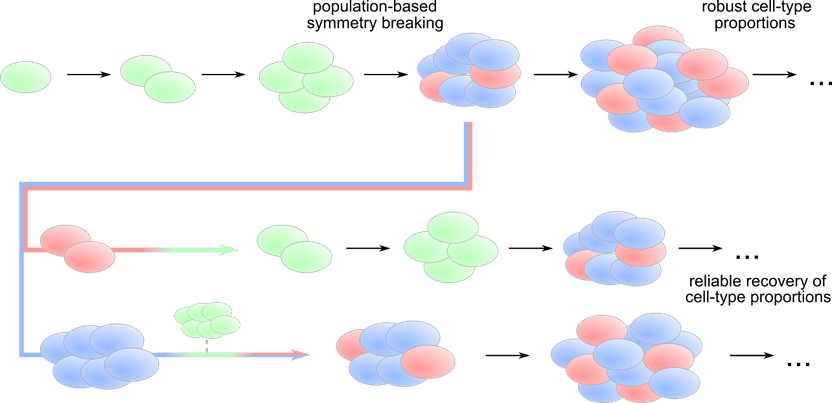
Dynamical model reveals how cell-cell communication in agrowing population can trigger differentiation and robust cell type proportions (top), but also recover the exact proportions (middle and bottom) if cell types are separated by perturbation.
MPI für molekulare Physiologie
Stem cells are the all-rounders among the cells in the body. They can differentiate into different cell types, such as skin cells, nerve cells or bone cells. Thus, during early embryonic development, a disordered bunch of stem cells transforms into ordered body structures. The information required for differentiation is stored in the genome of the stem cells. However, a blueprint for the formation of body structures is missing. Nevertheless, the development of different tissues must be executed with great precision and at the right time. How this complex process is coordinated still remains elusive.
Cells talk to each other
So far it has been assumed that the coordination of these processes takes place at the level of individual cells acting independently of one another. They receive signals from their environment that trigger the production of genetic markers and the development of characteristic gene expression patterns, and thereby stem cells differentiate into a cell with a specific function. In this framework however it is hard to explain how the right proportions of different cell types are generated, and how the timing of the differentiation emerges.
Aneta Koseska's group has now established a completely new theoretical concept to describe cellular development based on a population-level mechanism. With this changed view, the scientists can now describe how the correct timing of development into a organized structure can be guaranteed, and how development can proceed robustly and precisely despite disturbances. The scientists suggest that the growth of the cell community can drive the fate of individual cells and thereby offer a missing link between morphogenesis and pattern formation.
Theoretical concepts have a rich history in biology
These theoretical concepts are tested using mathematical models that capture the essential mechanisms and parameters of a biological process. Complex events in the cell can be thereby described and predictions can be made. These models can be used like artificial laboratories to validate hypotheses made from experimental data but also used in developing new hypothesis that can then be experimentally tested. "Such research seems very abstract, but theoretical ideas have a rich history in biology " explains Aneta Koseska. One of the most known examples is the evolutionary theory proposed by Darwin, that was later mathematically formulated by other scientist. A theory gives us a way to understand “How does it function, what is the mechanism?” A direct link between theory and experiments is however crucial, as both parts are fundamental to generate understanding of complex processes.
Cell-cell communication as a general property
Communication between cells also plays an important role in other important processes such as wound healing for example. This is because cells must also continuously react to their environment. “With our newly developed concept, we want to investigate this in detail in the future, both theoretically and experimentally," says Aneta Koseska.



















































