Medication-based starvation of cancer cells
Findings on cancer medication reveal protein regulation mechanism
Immunomodulatory drugs, including the Contergan derivatives lenalidomide and pomalidomide have significantly improved the therapy of hematologic malignancies such as multiple myeloma. Researchers at the Technical University of Munich (TUM) have now further decoded the mode of action in this class of medications. At the same time, they identified new innovative targeted cancer therapies.
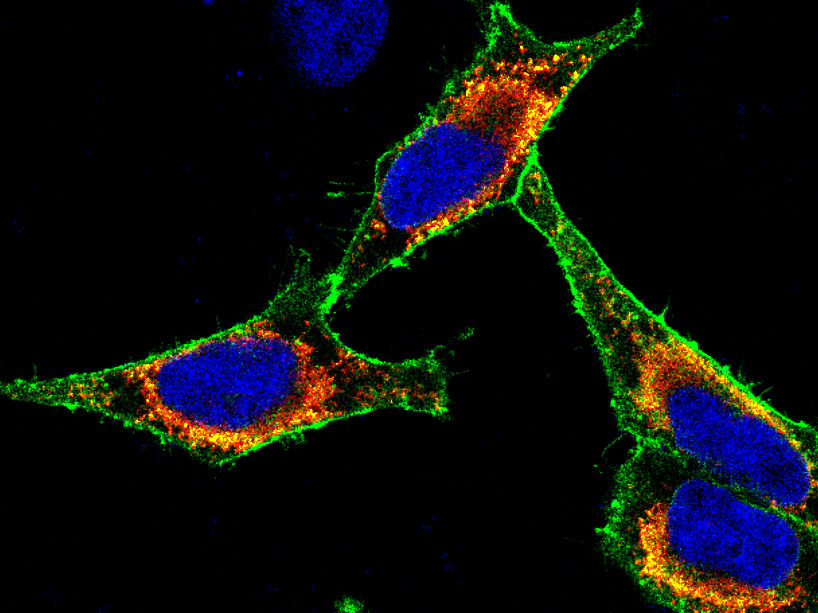
Immunofluorescence image of cancer cells in which a membrane protein has been labeled with a green fluorescent dye. The nucleus is marked with a blue dye.
F. Bassermann
The drug thalidomide was sold as a sedative under the trade name Contergan in the 1950s and 1960s. At the time, its side-effects triggered one of the largest pharmaceutical scandals in history: The medication was taken from the market after it became known that the use of Contergan during pregnancy had resulted in over 10,000 cases of severe birth defects.
Currently, the successor preparations lenalidomide and pomalidomide are prescribed under strict supervision by experienced oncologists – the active ingredients are a cornerstone of modern cancer therapies. The use of lenalidomide and pomalidomide has considerably improved the success rate of therapies and patient survival, particularly for hematological malignancies such as multiple myeloma. Since these substances can influence the immune system, they are referred to as immunomodulatory drugs (IMiDs).
Many membrane proteins affected
Previous studies have shown that IMiDs bind to a protein called cereblon, which results in the malfunction of a protein complex on the surface of tumor cells, thus inhibiting tumor growth. A research team led by Prof. Florian Bassermann and Vanesa Fernández of the university hospital Klinikum rechts der Isar of TUM has now deciphered the exact mechanism and the scope of this dysregulation in a new study.
They discovered that cereblon supports the protein HSP90 as what is known as a co-chaperone; HSP90 is responsible for the correct folding of thousands of proteins in human cells. The scientists were able to show that the support function of the co-chaperone cereblon is specific for membrane proteins. These proteins, which are anchored on the surface of a cell, are essential for tumor cells to grow: They enable cells to communicate with neighboring cells, they pass on growth signals and take in important nutrients.
Upon IMiD-treatment, cereblon can no longer bind to the HSP90 machinery, and as a result loses its supportive function in the quality control of membrane proteins. "Using proteome-wide analyses, we were able to show that a large number of essential proteins on the surface of cancer cells are destabilized by IMiD-treatment," says oncologist Florian Bassermann. "This ultimately explains the unusually broad effects of these substances."
Starving tumor cells
In multiple myeloma the proteins CD98hc and LAT1 are particularly affected. Together these proteins usually ensure that cancer cells are supplied with amino acids. Since cancer cells in the case of multiple myeloma have an especially high need for nutrients like amino acids, CD98hc and LAT1 are very abundant proteins in these cells. The research team has now shown that IMiD-treatment significantly reduces the uptake of essential amino acids and thus inhibits the growth of the tumor cells. "This literally starves out the cancer cells," explains Michael Heider, first author of the study.
New targeted therapeutic options
The discovery that multiple myeloma cells can be attacked by targeting the proteins CD98hc and LAT1 opens up new possibilities for innovative therapies in this currently incurable cancer. Together with Wolfgang Weber, TUM Professor for Nuclear Medicine, the researchers tested a molecule which is aimed at CD98hc, known as an anticalin. The molecule was recently developed by Arne Skerra, Professor for Biological Chemistry at TUM. The results show that the molecule binds specifically to the cell surface protein CD98hc in both cell culture and mouse models. This anticalin could therefore be used for targeted therapy and diagnosis in the future. "Early clinical studies to further evaluate the anticalin are already being planned," says Bassermann.
Original publication
Heider M., Eichner R., Stroh J., Morath V., Kuisl A., Zecha J., Lawatscheck J., Baek K., Garz A.K., Rudelius M., Deuschle F.C., Keller U., Lemeer S., Verbeek M., Götze K.S., Skerra A., Weber W.A., Buchner J., Schulman B.A., Kuster B., Fernández-Sáiz V., Bassermann F.; "The IMiD target CRBN determines HSP90 activity towards transmembrane proteins essential in multiple myeloma"; Molecular Cell; 2021