Fighting Cancer from a Chair
Platinum complex inhibits metastasis through conformational modulation of heparan sulfate
cisplatin has been used to treat cancer since the 1970s. Since then, many other platinum-containing cytostatic drugs have been developed, such as triplatinNC, a highly charged complex that contains three ligand-bridged platinum atoms. Unlike cisplatin, this drug also directly inhibits metastasis. The reason for this seems to be modulation of the geometry of a sugar component of heparan sulfate, an important component of the extracellular matrix, reports a research team in the journal Angewandte Chemie.
© Wiley-VCH
Heparan sulfate, a glycosaminoglycan, is a chain of ring-shaped sugar molecules. It is involved in many regulatory processes, as well as in the growth and metastasis of tumors. In order for a tumor to grow and form metastases, the extracellular matrix must be broken up in certain locations to allow cells to migrate. The splitting of heparan sulfate by the enzyme heparanase and the release of certain growth factors that bind to heparan sulfate play an important role in this process.
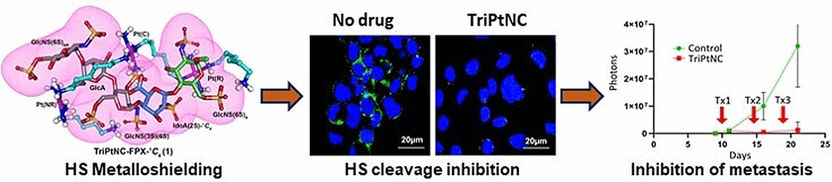
In order to shed light on the interaction of heparan sulfate with triplatinNC, a team led by Anil K. Gorle, Susan J. Berners-Price, and Nicholas P. Farrell at Griffith University (Brisbane, Australia), and Virginia Commonwealth University and Massey Cancer Center (Richmond, Virginia, USA), used fondaparinux (FPX), a molecule made of five sugar units, as a model for heparan sulfate. A combination of computer calculations and experimental data showed that triplatinNC changes the geometry of a specific sugar component of heparan sulfate (a sulfated iduronic acid). The six-membered ring of the iduronic acid can adopt two different spatial conformations: a chair form or a twist-boat form. In free FPX, the chair and twist-boat forms are in a 35:65 ratio. In the presence of triplatinNC, this shifts to 75:25. In the now preferred chair form, there is a pocket into which the platinum drug fits very well, allowing it to bind strongly. In actual heparan sulfate, the result of the strong bonding by triplatinNC is to effectively block it from being split by heparanase.
A tumor cell line in a synthetic extracellular matrix served as a model for triple-negative breast cancer, which is an aggressive form of cancer that is especially hard to treat. Treatment with heparinase initiated significant cell migration in the model. Prior treatment with triplatinNC significantly reduced cell migration—an effect not seen with cisplatin. The team was also able to confirm the anti-metastatic activity of triplatinNC in tests with mice.
TriplatinNC thus demonstrates dual activity. In addition to a cytotoxic effect caused by its action on DNA, it has an anti-metastatic effect caused by interference with the functionality of heparan sulfate. This opens new possibilities for the design of anti-metastatic platinum complexes.
Original publication
Most read news
Original publication
Dr. Anil K. Gorle et al.; "Conformational Modulation of Iduronic Acid‐Containing Sulfated Glycosaminoglycans by a Polynuclear Platinum Compound and Implications for Development of Antimetastatic Platinum Drugs"; Angewandte Chemie International Edition; 2020
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

FastTrack™ | UV/VIS spectrophotometers | Mettler-Toledo
Circadian clock protein revealed - Researchers learn how biological clocks tick by solving protein's structure