DESY’s X-ray source PETRA III points possible ways to better RNA vaccines
The pharmaceutical company BioNTech and the University of Mainz are conducting research with other partners on the EMBL beamline
Advertisement
The Mainz-based biotech company BioNTech, which recently presented the first promising results for a coronavirus vaccine together with the US company Pfizer, is already conducting research on the next generation of RNA drugs at DESY’s X-ray source PETRA III. Using the P12 beamline, operated by the European Molecular Biology Laboratory EMBL, BioNTech has been investigating, together with the Universities of Mainz, Tel Aviv and Leiden as well as the Research Centre Jülich and EMBL, how so-called messenger RNA (mRNA) can be packaged better so as to be more effective in the target organism. The researchers are reporting a number of results in three papers, published in the journals Applied Nano Materials, Cells and Langmuir. The papers also illustrate the potential of analytical research carried out with the help of the research infrastructure available on the DESY campus.
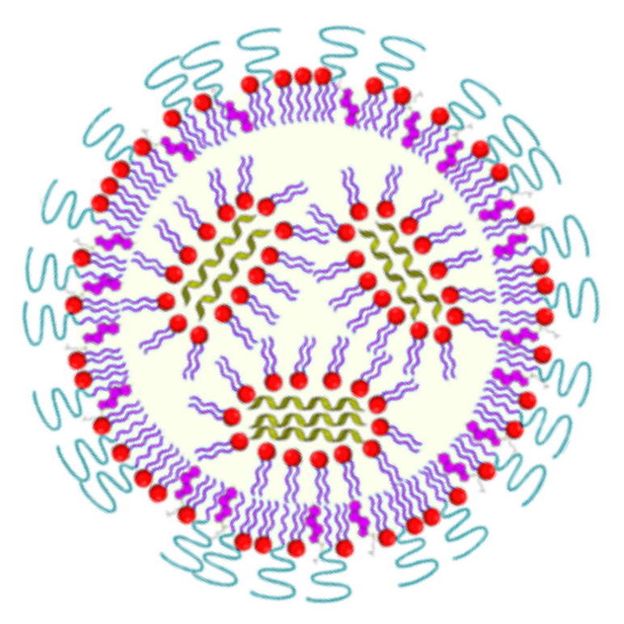
Schematic representation of a lipid nanoparticle loaded with mRNA (green).
BioNTech, Cristina Sala
The candidate vaccine developed by BioNTech and Pfizer, like the potential vaccine recently presented by the US company Moderna, belongs to the new class of mRNA vaccines, which are thought to have great potential not only for fighting viruses but also for personalised cancer treatments. This new technique involves introducing the blueprint for a key protein in a pathogen, a so-called antigen, into the cells of the subject in the form of mRNA, whereupon the cells then start to produce this key protein. The protein itself does not cause any illness, but the body’s immune system recognises it as being foreign and starts to make antibodies against it. If the body later encounters the actual pathogen, the immune system recognises the protein and is already prepared.
This method is very versatile, permitting a rapid response to the outbreak of an epidemic. It is also possible to make the body produce antigens to certain cancer cells, thereby triggering an immune response to these cancer cells. In principle, the blueprint for any protein can be introduced into the body: not only that of a pathogen or cancer cell, but also instructions for building proteins that can help to treat certain diseases. These instructions are usually encoded in the form of messenger RNA. Normally, the mRNA code is read from the DNA genome inside the cell and converted into proteins by the cell’s protein factories, the ribosomes. In the case of an RNA vaccine, ready-made mRNA is introduced into the cells from the outside and processed directly by the ribosome. The cell’s own genetic material, stored in the nucleus, remains unaltered and the mRNA that has been introduced is rapidly degraded, so that production of the pathogen’s protein soon comes to a standstill.
Introducing the mRNA into the cell is a major challenge, however. Pure mRNA would be degraded by the enzymes found throughout the tissue even before the cells could absorb it. Various strategies are therefore used to hide the messenger RNA inside nanoparticles and thus transport it inside the cells of the body. As a rule, the mRNA is introduced using nanoparticles made of certain polymers or so-called lipids, the molecules that make up cell membranes, for example.
If properly configured, the resulting nanoparticles are absorbed by the cells, where the mRNA is then released. The efficiency of this so-called transfection process is crucial for the effectiveness of an RNA vaccine. At PETRA III, scientists have been investigating various new approaches for packaging and delivering mRNA.
Among other things, the scientists have developed mRNA lipid nanoparticles (LNPs) that are tailor-made for this purpose, using a polymer made from the amino acid sarcosine, which is produced naturally by the body. Usually, polyethylene glycol (PEG), a polymer widely utilised in the pharmaceutical and cosmetic industries, is used for this purpose; but it has several disadvantages. The new type of LNPs led to a strong production of the desired protein while at the same time reducing unwanted immune responses, as the team now reports in Applied Nano Materials.
Researchers used small-angle X-ray scattering (SAXS) on the EMBL beamline P12 at PETRA III to investigate the internal organisation of these lipid nanoparticles and compared their effectiveness in different organs. In this way, they hope to identify those properties of the LNPs that are crucial for the uptake of RNA by specific cells. This will provide essential information for developing tailor-made particles for specific medical applications.
The scientists also used other lipid model systems to investigate how the internal structure of LNPs reacts to different pH values. This is important because changes in the pH play a key role in a crucial step in the absorption of the particles and their release into the cell. This is why so-called ionisable lipids are used to make the LNPs, whose charge changes depending on the pH. Adjusting the exact pH at which the charge changes is thought to be an essential basis for the high efficacy of LNPs.
Up until now, it has only been possible to study such changes that depend on the pH level indirectly, for example by spectroscopic methods. The team has now succeeded for the first time in directly demonstrating changes in the structure of model membranes containing the ionisable lipids. The research published in the journal Langmuir describes how the pH influences the internal structure of the nanoparticles. This knowledge can be used to develop optimised transport systems for transfecting specific types of cells, since the pH varies from one type of cell to the next.
The efficiency of mRNA transfection can also be increased by using the right combination of different materials to make the nanoparticles, as demonstrated in another research project. As the scientists involved write in the journal Cells, hybrid nanoparticles, containing both lipids and polymers suitably combined, achieved a significantly better transfection than pure lipid or pure polymer nanoparticles. Structural analyses, some of which were carried out on the EMBL’s P12 beamline at PETRA III, show that the particles with the highest transfection efficiency are characterised by a heterogeneous internal structure in which well-ordered and less well-ordered areas alternate in a characteristic pattern. Hence, they display a certain similarity to the composite materials studied by materials scientists.
All three approaches offer opportunities for achieving a better understanding of the fundamental principles governing the efficacy of mRNA-based drugs and thus for further improving future mRNA vaccines and optimising them individually for specific applications. Transfection is a decisive aspect for the success of mRNA vaccines, in which great hopes are currently being placed. In addition to BioNTech, Pfizer and Moderna, other companies such as Curevac, based in Tübingen, are also working on mRNA-based coronavirus vaccines. Although other aspects, such as long-term efficacy, still need to be investigated, this already gives some idea of the potential offered by this technology, which could in principle be used to very quickly develop vaccines against a range of infectious diseases, but also therapeutic vaccines for the treatment of existing diseases such as cancer.
The research shows the outstanding opportunities for research and experimentation that are available through the research infrastructure on the DESY campus, allowing progress to be made in innovation, technology and medicine. In times of crisis, like the current coronavirus pandemic, analytical research in particular is enormously relevant. Such analyses can lead to important findings which can be used to develop new active pharmaceutical ingredients. Their potential is far from being exhausted.
Original publication
"Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for mRNA"; Christian D. Siewert, Heinrich Haas, Vera Cornet, Sara S. Nogueira, Thomas Nawroth, Lukas Uebbing, Antje Ziller, Jozef Al-Gousous, Aurel Radulescu, Martin A. Schroer, Clement E. Blanchet, Dmitri I. Svergun, Markus P. Radsak, Ugur Sahin and Peter Langguth; Cells; 2020.
"Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery"; Sara S. Nogueira, Anne Schlegel, Konrad Maxeiner, Benjamin Weber, Matthias Barz, Martin A. Schroer, Clement E. Blanchet, Dmitri I. Svergun, Srinivas Ramishetti, Dan Peer, Peter Langguth, Ugur Sahin, and Heinrich Haas; Applied Nano Materials; 2020.
"Investigation of pH-Responsiveness inside Lipid Nanoparticles for Parenteral mRNA Application Using Small-Angle X‐ray Scattering"; Lukas Uebbing, Antje Ziller, Christian Siewert, Martin A. Schroer, Clement E. Blanchet, Dmitri I. Svergun, Srinivas Ramishetti, Dan Peer, Ugur Sahin, Heinrich Haas, and Peter Langguth; Langmuir; 2020.























































