Researchers Improve Neuronal Reprogramming by Manipulating Mitochondria
The replacement of lost neurons is a holy grail for neuroscience
A new promising approach is the conversion of glial cells into new neurons. Improving the efficiency of this conversion or reprogramming after brain injury is an important step towards developing reliable regenerative medicine therapies. Researchers at Helmholtz Zentrum München and Ludwig Maximilians University Munich (LMU) have identified a hurdle towards an efficient conversion: the cell metabolism. By expressing neuron-enriched mitochondrial proteins at an early stage of the direct reprogramming process, the researchers achieved a four times higher conversion rate and simultaneously increased the speed of reprogramming.
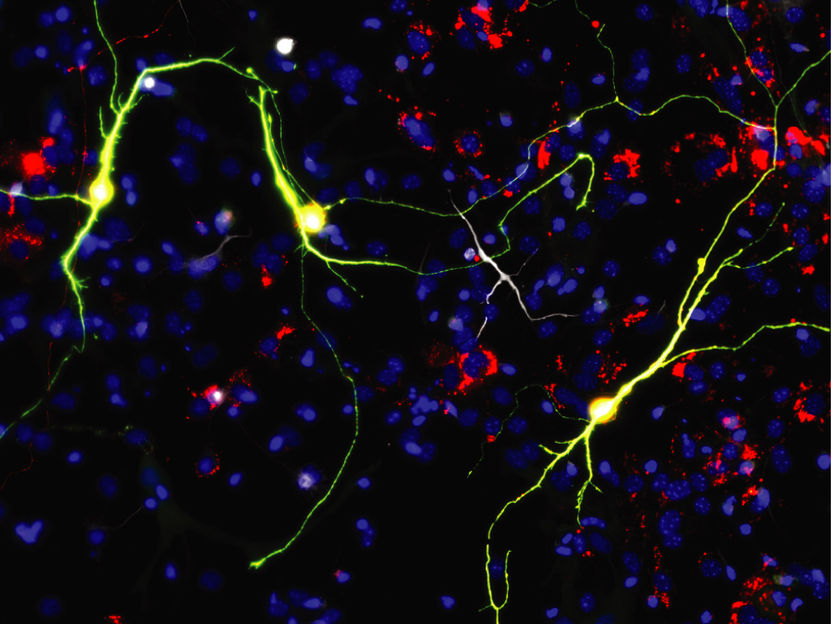
Reprogrammed neurons upon Ascl1 expression and neuron-enriched mitochondrial proteins.
© Helmholtz Zentrum München
Neurons (nerve cells) have very important functions in the brain such as information processing. Many brain diseases, injuries and neurodegenerative processes, are characterized by the loss of neurons that are not replaced. Approaches in regenerative medicine therefore aim to reconstitute the neurons by transplantation, stem cell differentiation or direct conversion of endogenous non-neuronal cell types into functional neurons.
Researchers at Helmholtz Zentrum München and LMU are pioneering the field of direct conversion of glial cells into neurons which they have originally discovered. Glia are the most abundant cell type in the brain and can proliferate upon injury. Currently, researchers are able to convert glia cells into neurons – but during the process many cells die. This means that only few glial cells convert into functional nerve cells, making the process inefficient.
Exploring new approaches
Magdalena Götz and her team investigated potential hurdles in the conversion process and took a new route: While most studies have focused on the genetic aspects of direct neuronal reprogramming, they decided to study the role of mitochondria and cell metabolism in this process. This was inspired by their previous work in collaboration with Marcus Conrad’s group at Helmholtz Zentrum München showing that cells die due to excessive reactive oxygen species in the conversion process.
“We hypothesized that if we were able to help reprogramming the metabolism of glia cells towards the metabolism of a neuron, this could improve the conversion efficiency”, explains Gianluca Russo, first-author of the study. Given their previous data, the researchers focused on mitochondria, the cell’s powerhouse. The group extracted mitochondria from neurons and astrocytes (a specific type of glia cell) of mice and compared them by studying their proteins in collaboration with Stefanie Hauck’s group of proteomic experts at Helmholtz Zentrum München. Surprisingly, they found that mitochondria of neurons and astrocytes differ in 20 percent of their proteome. This means that between astrocytes and neurons every fifth mitochondrial protein is different.
Reprogrammed neurons activate neuron-enriched mitochondrial proteins at a late stage
“Knowing how different the mitochondrial proteome of neurons is from astrocytes, we needed to see if and when neurons converting from astrocytes actually acquire the mitochondrial proteome of a neuron or not”, says Giacomo Masserdotti, co-last author of the study. In a standard reprogramming process, glia cells like astrocytes convert to neurons within a few days and develop into functional neurons within two weeks. “It was striking that cells showed mitochondrial proteins, which are typical for neurons, relatively late in the reprogramming process, only after one week. Since most cells die before this time, this could be a hurdle. In addition, cells that failed to be reprogrammed, still expressed astrocyte-enriched mitochondrial proteins.” With this new insight, the researchers hypothesized that the failure of turning on neuronal mitochondrial proteins may be blocking the conversion process.
Improving and accelerating the conversion through metabolism
To overcome this hurdle, the group employed CRISPR/Cas9 technology in close cooperation with Stefan Stricker’s and Wolfgang Wurst’s groups at Helmholtz Zentrum München. With new gene activation tools developed by this group, neuron-enriched mitochondrial proteins could be activated at an early stage of the reprogramming process of astrocytes to neurons. By manipulating one to two mitochondrial proteins only, the researchers gained four times more reprogrammed neurons. On top of that, the neurons appeared and matured faster, as revealed by continuous live imaging.
“I was amazed that changing the expression of few mitochondrial proteins actually drives the speed of reprogramming”, says Magdalena Götz, the lead author of the study. “This shows how important the cell-type-specific differences of mitochondrial proteins are. And indeed, together with our proteome experts at Helmholtz Munich, we are discovering further organellar differences between cell types that reach up to 70 percent. This will pave the way to further improve the reprogrammed neurons to resemble as much as possible endogenous neurons also after brain injury in vivo.”