Researchers create artificial cell organelles for biotechnology
Synthetic vesicles are mini-laboratories for customised molecules
Advertisement
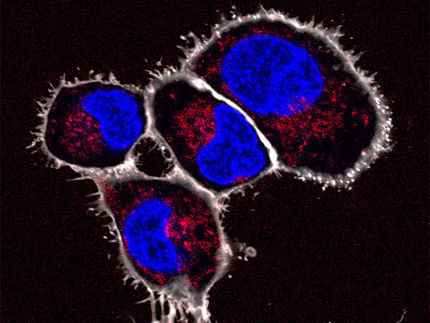
Cells of higher organisms use cell organelles to separate metabolic processes from each other. This is how cell respiration takes place in the mitochondria, the cell’s power plants. They can be compared to sealed laboratory rooms in the large factory of the cell. A research team at Goethe University has now succeeded in creating artificial cell organelles and using them for their own devised biochemical reactions.
Symbolic image
Mahmoud-Ahmed, pixabay.com
Biotechnologists have been attempting to “reprogram” natural cell organelles for other processes for some time – with mixed results, since the “laboratory equipment” is specialised on the function of organelles. Dr Joanna Tripp, early career researcher at the Institute for Molecular Biosciences has now developed a new method to produce artificial organelles in living yeast cells.
To this end, she used the ramified system of tubes and bubbles in the endoplasmic reticulum (ER) that surrounds the nucleus. Cells continually tie off bubbles, or vesicles, from this membrane system in order to transport substances to the cell membrane. In plants, these vesicles may also be used for the storage of proteins in seeds. These storage proteins are equipped with an “address label” – the Zera sequence – which guides them to the ER and which ensures that storage proteins are “packaged” there in the vesicle. Joanna Tripp has now used the “address label” Zera to produce targeted vesicles in yeast cells and introduce several enzymes of a biochemical metabolic pathway.
This represents a milestone from a biotechnical perspective. Yeast cells, the “pets” of synthetic biology not only produce numerous useful natural substances, but can also be genetically changed to produce industrially interesting molecules on a grand scale, such as biofuels or anti-malaria medicine.
In addition to the desired products, however, undesirable by-products or toxic intermediates often occur as well. Furthermore, the product can be lost due to leaks in the cell, or reactions can be too slow. Synthetic cell organelles offer remedies, with only the desired enzymes (with “address labels”) encountering each other, so that they work together more effectively without disrupting the rest of the cell, or being disrupted themselves.
“We used the Zera sequence to introduce a three-stage, synthetic metabolic pathway into vesicles,” Joanna Tripp explains. “We have thus created a reaction space containing exactly what we want. We were able to demonstrate that the metabolic pathway in the vesicles functions in isolation to the rest of the cell.”
The biotechnologist selected an industrially relevant molecule for this process: muconic acid, which is further processed industrially to adipic acid. This is an intermediate for nylon and other synthetic materials. Muconic acid is currently won from raw oil. A future large-scale production using yeast cells would be significantly more environment-friendly and sustainable. Although a portion of the intermediate protocatechuic acid is lost because the vesicle membrane is porous, Joanna Tripp views this as a solvable problem.
Professor Eckhard Boles, Head of the Department of Physiology and Genetics of Lower Eukaryotes observes: “This is a revolutionary new method of synthetic biology. With the novel artificial organelles, we now have the option of generating various processes in the cell anew, or to optimise them.” The method is not limited to yeast cells, but can be utilised for eukaryotic cells in general. It can also be applied to other issues, e.g. for reactions that have previously not been able to take place in living cells because they may require enzymes that would disrupt the cell metabolic process.