Photopharmacology - A light-trigger for the proteasome
A light-sensitive inhibitor can control cell division and cell death – and provides a promising approach for studies of essential cellular processes and the development of novel tumor therapies
The ability to precisely control biological and chemical processes is an essential element of both basic research and medicine. Light represents an attractive stimulus in this context, as its effects can be accurately modulated both spatially and temporally. These desirable properties are the reason why the development of light-controllable molecules has become such an important goal for biological chemists. Such tools promise to make significant contributions to the elucidation of basic cellular functions, the detailed understanding of medical disorders and the design of new therapeutic strategies to combat them. A group of researchers led by cell biologist Esther Zanin at LMU‘s Biocenter, in cooperation with the chemist Henry Dube (who moved in April of this year from the LMU to the University of Erlangen-Nürnberg) has now developed a light-sensitive chemical inhibitor, that allows them to control two fundamental cellular processes, cell division and cell death, with light.
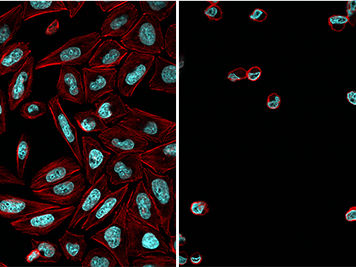
Cells treated with the light-dependent inhibitor (actin cytoskeleton in red, DNA in blue) after exposure to blue light (right) and without activating blue light radiation (left).
Esther Zanin
Cell division is a vital and highly complex process. It is therefore subject to tight regulation to ensure that cells divide only at the right time and error free. Defective cells are eliminated by programmed cell death (also known as ‘apoptosis’). Both correct cell division and the disposal of defective cells depend on a molecular machine called the proteasome, which specifically degrades cellular proteins that are either damaged or no longer required.
“We have now modified an established and versatile chemical inhibitor of the proteosome by adding a light-sensitive protective group to it,” says Zanin. “This group blocks the reactive aldehyde function of the inhibitor and prevents it from binding to the proteasome.” In the dark, the inhibitor is therefore inactive and the proteasome functions normally. However, exposure of the cells to blue light detaches the protective group, thus allowing the inhibitor to interact with the proteosome and inhibit its function. Since the activating blue light radiation can be accurately targeted, the action of the inhibitor can be very precisely controlled. “By this means, we are able to arrest the division of tumor cells at a specific stage of the process, and to trigger apoptosis in a targeted manner,” Zanin explains.
She and her colleagues believe that the new light-sensitive proteosome inhibitor will prove to be a valuable tool for the study of a wide range of dynamic cellular processes – for example, in the context of development, during which cells and tissues undergo rapid and often radical changes during a short time and at confined locations. In addition, proteosome inhibitors have promising applications as therapeutic agents – in the treatment of cancer, for instance. “The ability to activate these compounds specifically in both time and space could make them more efficacious in the future, while reducing the incidence of side-effects,” says Zanin. However, reaching this goal will require further work, as the inhibitor employed in the new study is not suitable for medical use in its present form.