A novel approach to the development of antibiotics, immunotherapy medications and anti-cancer drugs
ABC transporters play an essential, hitherto unknown role in the biosynthesis of NRPS products
antibiotics as well as important drugs for the treatment of cancer and immune diseases are produced by microorganisms through multi-modular enzyme complexes called non-ribosomal peptide synthetases (NRPSs) and polyketide synthetases (PKSs). A research team led by Vetmeduni Vienna recently revealed that ABC transporters play an essential, hitherto unknown role in the biosynthesis of NRPS products. The genetic and biochemical data presented in the journal mBio provide information about a new, significant aspect of the biosynthesis of secondary microbial metabolites that are important for human and veterinary medicine – with practical relevance for the development of novel drug therapies.
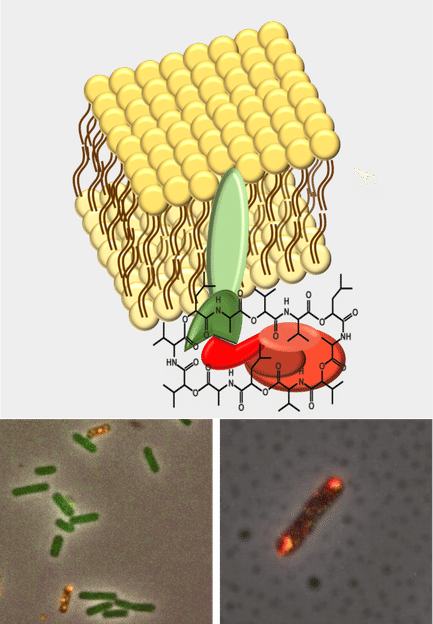
Model of the cell membrane with inserted ABC transporter CesCD (green) and attached NRPS CesAB (red) leading to cereulide synthesis. Bottom panel: Merged fluorescent microscopy images (yellow) of biosynthetic complex CesC and CesA .
Agnieszka Gacek-Matthews
Non-ribosomal peptide synthetases (NRPSs) play a key role in the production of bioactive natural products. NRPSs are multi-modular bacterial and fungal megaenzymes responsible for the synthesis of various bioactive natural compounds, such as antibiotics (e.g. penicillin and vancomycin), anti-tumour drugs (e.g. bleomycins and cryptophycins), immunosuppressants (e.g. cyclosporine A) and toxins (e.g. microcystins and cereulide). It is therefore important to understand the molecular mechanisms and complex architecture of the cellular NRPS machineries responsible for the synthesis of these biomedical and pharmaceutical important microbial compounds.
ABC transporters: essential for the biosynthesis of the depsipeptide cereulid
In this study led by Monika Ehling-Schulz, head of the Institute of Microbiology at Vetmeduni Vienna, an international research team succeeded in showing that an ABC transporter is an essential and integral part of the non-ribosomal peptide biosynthesis machinery cereulide (Ces) synthetase. The Ces synthetase of the emetic bacterium Bacillus cereus is responsible for the production of the depsipeptide toxin cereulide that causes food poisoning.
The researchers demonstrated that tethering of CesAB synthetase to the cell membrane by the ABC transporter CesCD is critical for the assembly of cereulide. In vivo studies revealed that CesAB co-localizes with CesCD on the cell membrane, which suggests a direct involvement of this ABC transporter in the biosynthesis of a non-ribosomal peptide. “Our experiments show that mutation of cesCD resulted in decreased interaction of CesCD and CesAB, which negatively affected cereulide biosynthesis. We were also able to identify specific domains within CesAB synthetase interacting with CesC, thus providing a first look at the molecular mechanisms underlying the interaction between an ABC transporter and a NRPS,” says first author Agnieszka Gacek-Matthews. The researchers also demonstrated that a structurally similar ABC transporter from another bacterium can assume the function of the B. cereus ABC transporter CesCD in cereulide biosynthesis, suggesting that the direct involvement of the ABC transporter in secondary metabolite biosynthesis, described here for the first time, could be a widespread mechanism.
Enormous relevance for the search for new drug therapies
According to Ehling-Schulz, the new insights into natural product biosynthesis may facilitate the discovery of new metabolites with bioactive potential. The present study can help to better understand the biosynthesis of microbial secondary metabolites and provides evidence for an essential function of ABC transporters in natural product synthesis. “We expect these findings to be of high relevance for the understanding of the architecture and cellular organization of NRPS multi-enzyme machinery and that they may provide a foundation for future work that could contribute to the discovery of novel bioactive microbial products,” says Ehling-Schulz. One possible benefit could be the discovery of novel therapeutics against pan-resistant microbes. “The function of the investigated ABC transporters in cereulide biosynthesis described in our study may represent an important new approach to optimizing the use of non-ribosomal natural products,” Ehling-Schulz points out.




















































