Spin-off project successfully developed a COVID-19 vaccine candidate
Quick, efficient and scalable vaccine manufacturing: virus-like particle developed and produced
With the current pandemic crisis, the lack of worldwide vaccine manufacturing capacity has become a serious concern. ContiVir, a spin-off project at the Max Planck Institute for Dynamics of Complex Technical Systems Magdeburg, has successfully produced a virus-like particle as a Covid-19 vaccine candidate, using a fully scalable system for quick vaccine manufacturing in large scales. The technologies and processes have been developed by Dr.-Ing. Felipe Tapia und Dr.-Ing. Pavel Marichal-Gallardo, members of the Bioprocess Engineering group (headed by Prof. Dr.-Ing. Udo Reichl).
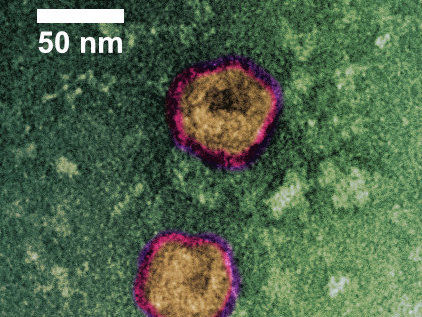
The Corona virus-like particles (VLPs) shown in the image were purified using ContiVir’s innovative steric exclusion technology.Transmission Electron Microscopy (TEM) with negative staining.
© Max-Planck-Institut Magdeburg
The biosafety levels required for a laboratory to handle the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) are very high. This seriously limits the number of facilities that can work on vaccines.
The scientists of the ContiVir team at the Max Planck Institute for Dynamics of Complex Technical Systems have designed and produced a vaccine candidate called a virus-like particle (VLP). VLPs resemble the morphology and structure of SARS-CoV-2 particles but do not contain any of its infective genetic material. Dr. Pavel Marichal-Gallardo, responsible for Downstream Processing, says that this makes the particles „completely safe and suitable for use in any biolaboratory.“
The corona VLPs were purified using ContiVir’s innovative membrane-based chromatographic technique (Steric Exclusion Chromatography SXC). This method can be used to concentrate and purify a wide variety of viruses with high product yields using very similar process conditions supporting short process development.
Dr. Julian Lopez, who is in charge of ContiVir’s Business Development, said: “The fact that we could produce a vaccine candidate for a new disease in just a few weeks demonstrates the potential of our technology. About half of the vaccine candidates against COVID-19 currently listed by the World Health Organization can be produced and purified using our system, which is fully scalable to industrial level. Several biopharmaceutical companies, including some of the leaders in the field, have already expressed interest in our technologies, and we have recently started providing them with prototypes for evaluation.”
There are currently hundreds of vaccine candidates, and a promising handful of them are in late-stage clinical testing. However, manufacturing the billions of vaccine doses that will be needed is a separate challenge. “Our technologies are more than 10 times as efficient as current manufacturing systems.”, says Dr. Felipe Tapia, responsible for Upstream Processing. “They can therefore provide more material for analytics or pre-clinical testing, and they have the potential to mitigate vaccine manufacturing shortages and long waiting times that might ultimately threaten the most vulnerable populations.”
The research of ContiVir is funded by the European Union and the German federal government within its program EXIST Forschungstransfer. The labs of ContiVir are located at the Chair for Bioprocess Engineering at the Otto von Guericke University Magdeburg.
Original publication
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.
























































