Structure of ATPase, the world’s smallest turbine, solved
Location of the permeability transition pore found
The chemical ATP, adenosine triphosphate, is the fuel that powers all life. ATP is found in all known forms of life, where it provides energy to drive muscle contraction, impulse propagation and chemical synthesis. Despite ATP’s central role, the structure of the enzyme generating ATP, F1Fo-ATP synthase, in mammals, including humans, has not been known so far. Now, Leonid Sazanov and his group at the Institute of Science and Technology Austria (IST Austria) report the first complete structure of the mammalian F1Fo-ATP synthase. This structure also settles a debate on how the permeability transition pore, a structure involved in cell death, cancer, and heart attacks, forms.
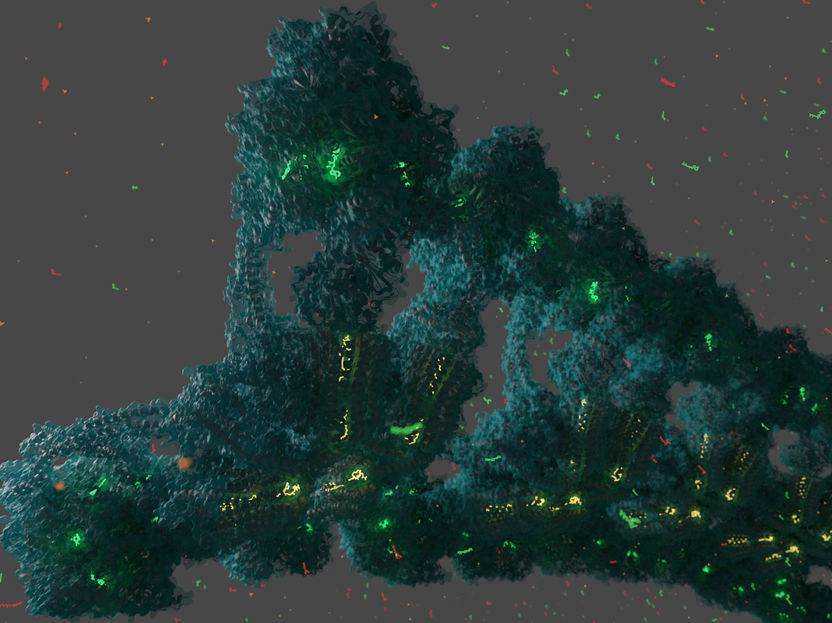
Rows of ATP synthase as they would form on the ridges of mitochondrial membranes. ATP, ADP and lipids are shown in bright colours.
Gergely Pinke / IST Austria
ATP synthase is also referred to as complex V of the respiratory chain, a series of protein complexes in the membrane of mitochondria. This respiratory chain creates a proton gradient, which the ATP synthase uses to make ATP. Previously, Sazanov was the first to solve the protein structure of bacterial complex I, and the first to solve the structure of a mammalian complex I. In the new study, Sazanov and lab members Gergely Pinke and Long Zhou turned to mammalian complex V, the final unsolved structure in the mammalian respiratory chain. “F1Fo-ATP synthase is one of the most important enzymes on Earth. It provides energy for most life forms, including us humans, but until now, we didn’t know fully how it works”, explains Sazanov.
Rotation muddies the picture
As the structure of the mushroom-like F1 soluble domain is known already, Sazanov and his team looked particularly at the Fo domain, embedded in the mitochondrial membrane. Here, protons are translocated at the interface between the so-called c ring, a ring made up of identical protein subunits, and the rest of Fo. Protons are moved across the membrane as each c subunit picks up a proton on one side of the membrane, rotates with the ring, and releases the proton on the other side. This c-ring is attached to the central shaft of F1 and its rotation generates ATP within F1. To solve the structure of the Fo domain and the entire complex, the researchers studied the enzyme from sheep mitochondria using cryo-electron microscopy. And here, ATP synthase poses a special problem: because it rotates, ATP synthase can stop in three main positions, as well as in substates. “It is very difficult to distinguish between these positions, attributing a structure to each position ATP synthase can take. But we managed to solve this computationally to build the first complete structure of the enzyme”, Sazanov adds.
Location of the permeability transition pore found
In their high-resolution structure of Fo, the researchers found that the c-ring is plugged by two lipids, one from each side of the membrane. While the top (facing F1) lipid rotates along with the shaft, the bottom lipid does not rotate, as it is likely connected to the Fo domain via a “hook apparatus”.
This newly uncovered structure sheds light on a controversy in biology: how and where the so-called permeability transition pore opens. This pore is linked with cell death, and opens for example during strokes and heart attacks. So far, it was known that the pore forms in mitochondria in response to high levels of Calcium, but the pore’s exact location remained unknown. Now, using the fully solved structure of F1Fo, Sazanov and his group can describe how the pore forms in F1Fo-ATP synthase: When Calcium binds in the F1 subunit, a large conformational change is induced. The complex has to accommodate this change, and in doing so, pulls on the hook apparatus. The apparatus in turn pulls out the lipid plug on the bottom side of the Fo, initiating pore opening. “When the pore is open for a longer period of time, the c-ring is destabilized and pore formation becomes irreversible”, explains Sazanov. “This model is consistent with all available data from mutants. To be fully sure that this is how the permeability transition pore forms, one would need to solve the structure of ATP synthase during Calcium-induced transitions, which we are doing now.”