More flexible than anticipated
New findings on the spike protein of SARS-CoV-2
Advertisement
To combat the coronavirus SARS-CoV-2, research has been stepped up in search of new vaccines and treatments. To enter the cells, the virus needs the spike protein on its surface. Researchers of the Paul-Ehrlich-Institut in co-operation with some research groups in Germany (EMBL at Heidelberg, MPI for Biophysics at Frankfurt) have analysed the spike protein in its natural environment using high resolution imaging and computer-assisted methods. In doing so, they have discovered a degree of mobility of the spikes on the virus surface not previously expected. The results are published in Science in its online edition of 18 August 2020
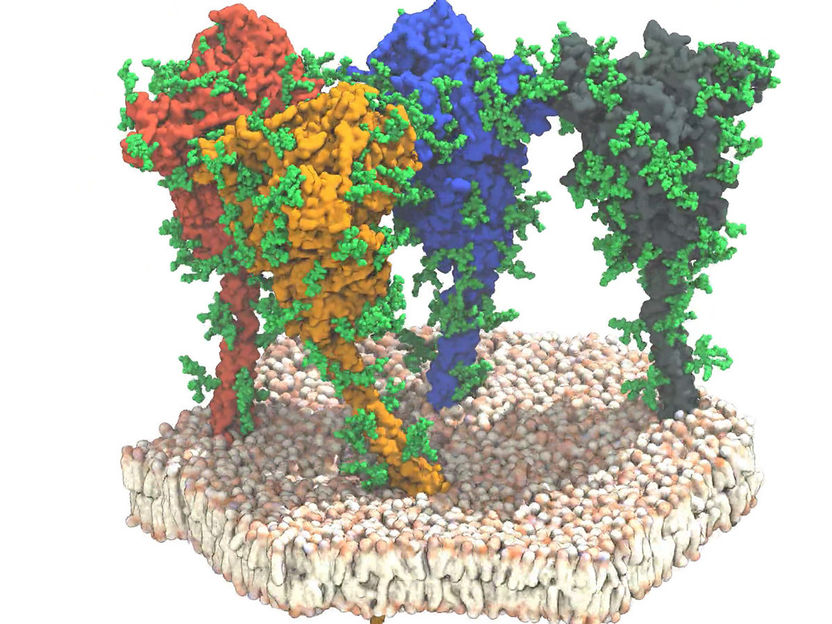
Simulation of four spike proteins (red, orange, blue and grey) of the SARS-CoV-2 virus. The proteins and lipids are shown in surface representation. The protective glycan chains are shown in green.
MPI for Biophysics, Frankfurt/Main
The coronavirus SARS-CoV-2 needs the spike proteins (S) on its surface to bind to specific receptors on the surface of human cells. It uses this pathway to infect the cells. These spike-shaped structures are in the focus of vaccine development since their role is to act as antigen to induce an immune response in humans that protects them from COVID-19. Researchers are studying the virus intensively, in particular, its surface structure, to gain insights into both the vaccines and the development of efficacious therapeutics for the treatment of infected patients. In this context, the knowledge of the spatial structures plays an important part, which are important for various processes such as entering the target cells.
The combination of the most modern technologies of cryo-electron tomography, sub-tomogram averaging, and molecular dynamics simulation allow the structural analysis of molecular structures in their natural environment at near-atomic resolution. Professor Jacomine Krijnse Locker, head of the Loewe Research group “Electron Microscopy of Pathogens“ and her research team at the Paul-Ehrlich-Institut, in co-operation with the section at the Paul-Ehrlich-Institut of which Priv Lect Michael Mühlebach as its head, and researchers of the European Laboratory of Molecular Biology (EMBL) the Max-Planck-Institut for Biophysics and the Institut for Biophysics of the Goethe University at Frankfurt/Main have analysed the spike protein in its natural environment by means of imaging methods and simulation models. For this purpose, they used SARS-CoV-2 particles extracted from the supernatant of infected cells. With the aid of the most modern electron microscopy methods at EMBL, 266 cryotomography images of around 1000 different viruses were generated, each bearing 40 spikes on their surfaces on average. In using sub-tomography averaging and image processing, important information on the structures of altogether 40,000 spikes could be obtained.
The result is favourable for the development of a vaccine. The upper sphere-shaped or v-shaped part of the spike, under natural conditions, shows a structure, which can be easily reproduced by recombinant proteins usable for vaccine development. The findings on the shaft used to fix the spherical part of the spike to the virus surface, however, were new.
Little was previously known about this structure. Now, the researchers have discovered that the shaft is extremely flexible. The images showed that it was seldom perpendicular to the membrane but tilted to all directions.
The team identified four different domains (sections) on the shaft, which they called the “hip”, “knee”, and “ankle” domain, and finally the foot domain, which is embedded in the membrane. Using a combination of molecular dynamic simulations and cryo-tomography, the researchers provided proof that these domains can execute bending movements. The data show that the spherical part of the spikes, which contains the receptor binding area and the components required for the target cell, is connected to a flexible shaft. “Like a balloon on a string, the spikes seem to move on the surface of the virus, which enables them to find the receptor for adhering to the target cell,” as Professor Jacomine Krijnes Locker describes the results. Finally, the analyses show that the shaft is equipped with many glycan chains. This could provide the shaft with a protective mantle, which will screen it from neutralising antibodies. This can be clarified in further experiments and will contribute to the understanding of immunological properties of the spike proteins on the road to effective vaccines.
Original publication
Turoňová* B, Sikora* M, Schürmann* C, Hagen WJH, Welsch S, Blanc FEC, Sören von Bülow S, Gecht M, Bagola K, Hörner C, v Zandbergen G, Mosalaganti S, Schwarz A, Covino R, Mühlebach MD, Hummer G, Krijnse Locker J, Beck M; "In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges"; Science; 2020 (*contributed in equal parts)




























































