Gene silencing may be responsible for induced pluripotent stem cells' limitations
Better understanding of mechanisms involved should improve reprogramming of embryonic-like cells
Scientists may be one step closer to being able to generate any type of cells and tissues from a patient's own cells. In a study that will appear in Nature, investigators from the Massachusetts General Hospital Center for regenerative medicine (MGH-CRM) and the Harvard Stem Cell Institute (HSCI), describe finding that an important cluster of genes is inactivated in induced pluripotent stem cells (iPSCs) that do not have the full development potential of embryonic stem cells. Generated from adult cells, iPSCs have many characteristics of embryonic stem cells but also have had significant limitations.
"We found that a segment of chromosome 12 containing genes important for fetal development was abnormally shut off in most iPSCs," says Konrad Hochedlinger, PhD, of the MGH-CRM and HSCI, who led the study. "These findings indicate we need to keep improving the way we produce iPSCs and suggest the need for new reprogramming strategies."
Although iPSCs appear quite similar to embryonic stem cells and give rise to many different types of cells, they have important limitations. Several molecular differences have been observed, particularly in the epigenetic processes that control which genes are expressed, and procedures that are able to generate live animals from the embryonic stem cells of mice are much less successful with iPSCs.
Previous studies have compared iPSCs generated with the help of viruses, which can alter cellular DNA, to embryonic stem cells from unrelated animals. To reduce the chance that the different sources of the cells were responsible for observed molecular differences, the MGH/HSCI research team prepared two genetically matched cell lines. After generating mice from embryonic stem cells, they used a technique that does not use viruses to prepare lines of iPSCs from several types of cells taken from those animals. They then compared the iPSCs with the original, genetically identical embryonic stem cells.
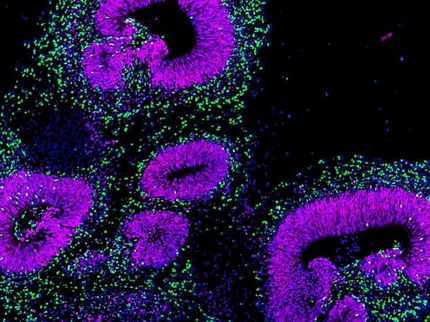
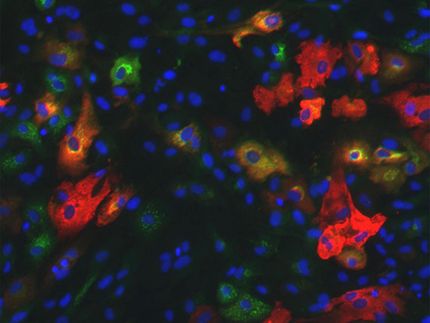
The most stringent assay of cells' developmental potential showed that two tested lines of embryonic stem cells were able to generate live mice as successfully as in previous studies, but no animals could be generated from genetically matched iPSCs. Closely comparing RNA transcription profiles of several matched cell lines revealed significantly reduced transcription of two genes in the iPSCs. Both genes are part of a gene cluster on chromosome 12 that normally is maternally imprinted – meaning that only the gene copies inherited from the mother are expressed.
Examination of more than 60 iPSCs lines developed from several types of cells revealed that this gene cluster was silenced in the vast majority of cell lines. While the gene-silenced iPSCs were able to generate many types of mouse tissues, their overall developmental potential was limited. In an assay that produces chimeric animals that incorporate cells from two different stem cells, mice produced from gene-silenced cells had very few tissues that originated from the iPSCs. However, in a few iPSC lines the gene cluster was normally activated, and in the most rigorous developmental assay, those iPSCs were as successful in producing live animals as embryonic stem cells have been. The authors believe this is the first report of animals being produced entirely from adult-derived iPSCs.
"The activation status of this imprinted cluster allowed us to prospectively identify iPSCs that have the full developmental potential of embryonic stem cells," says Matthias Stadtfeld, PhD, of the MGH-CRM and HSCI, a co-lead author of the report. "Identifying pluripotent cells of the highest quality is crucial to the development of therapeutic applications, so we can ensure that any transplanted cells function as well as normal cells. It's going to be important to see whether iPSCs derived from human patients have similar differences in gene expression and if they can be as good as embryonic stem cells – which continue to be the gold standard – in giving rise to the 220 functional cell types in the human body."
Hochedlinger adds, "Previous studies in mice have shown that embryonic stem cells derived from nuclear transfer – the technique used to clone animals – are indistinguishable from stem cells derived from fertilized embryos. Nuclear transfer is another way of reprogramming adult cells into embryonic-like cells, and comparing that approach with iPSC generation may yield important insights into ways of producing the safest and highest quality pluripotent cells for use in patients." Hochedlinger is an associate professor in the Harvard University Department of Stem Cell and Regenerative Medicine.