Researchers describe structure of SARS-CoV-2 proteins suitable for design of new drugs
COVID-19 has changed the lives of millions and even billions of people around the world. The disease is caused by SARS coronavirus 2 (SARS-CoV-2), an RNA virus, i.e. a virus that uses RNA to store its genetic information. In order to confront it, we must have a detailed understanding of the structure and function of its individual proteins.
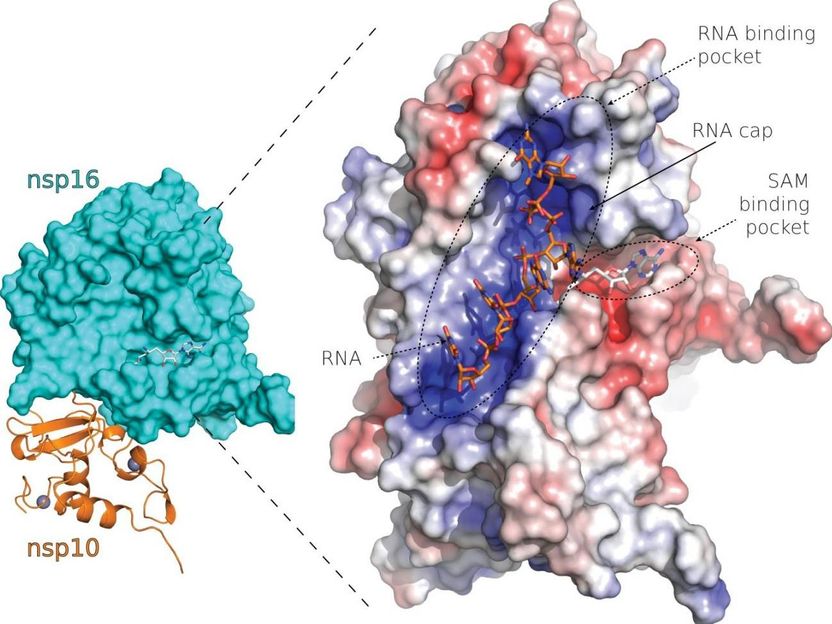
SARS-CoV-2 nsp10-nsp16 protein complex
Petra Krafcikova / IOCB Prague
One of the mechanisms that the coronavirus uses to try and outsmart our immunity and convince our cells that the viral RNA is harmless is the installation of so-called caps, special structures at the beginning of the RNA, thanks to which the viral RNA imitates human RNA, allowing the virus to infect the human body and multiply within it.
The cap installation process catalyzes the Nsp16 coronavirus protein with involvement from another viral protein, Nsp10. Headed by Dr. Evžen Bouřa and Dr. Radim Nencka, a group of researchers at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences used X-ray crystallography to determine and analyze the precise structure of the complex of these two proteins.
This allowed the researchers to identify several fundamental characteristics of the Nsp16 and Nsp10 protein complex, namely a deep canyon on the surface of the protein complex where binding of the viral RNA occurs and a cap is installed. This canyon can be targeted by inhibitors that suppress the activity of the Nsp16 and Nsp10 protein complex and thus of the entire cap installation process and may, in the future, serve as drugs to combat many coronaviruses.
“Hundreds of research teams tried to shed light on how the COVID-19 virus is able to hide its RNA from cellular immunity. In the end, two American teams and we here in Prague were the ones who succeeded,” explains Evžen Bouřa, head of the Structural Membrane Biology group.
“We used X-ray analysis to determine the structure of the responsible viral enzyme with inhibitor.”
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.