How smart, ultrathin nanosheets go fishing for proteins
Faster and simpler production of high-resolution, three-dimensional electron microscopy images of biomolecules
An interdisciplinary team from Frankfurt and Jena has developed a kind of bait with which to fish protein complexes out of mixtures. Thanks to this “bait”, the desired protein is available much faster for further examination in the electron microscope. The research team has christened this innovative layer of ultrathin molecular carbon the “smart nanosheet”. With the help of this new development, diseases and their treatment with drugs can be better understood, for example.
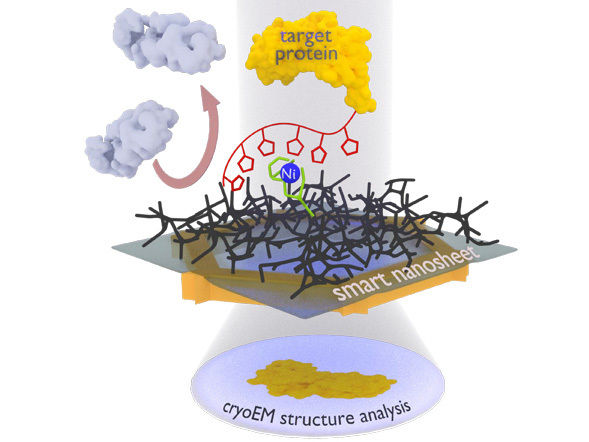
The new nanosheet process: The protein complex to be examined (yellow) is attached to the smart nanosheet via a nickel complex with the aid of a marker (red chain with pentagons). Unwanted proteins (gray) are repelled by the hydrogel (black grid). After freezing the entire structure, including a thin film of water, this can be irradiated with electrons to obtain images of the bound proteins, from which a computer can then calculate the 3D structure of the protein.
“With our process, new types of proteins can be isolated from mixtures and characterized within a week,” explains Daniel Rhinow from the Max Planck Institute of Biophysics in Frankfurt. “To date, just the isolation of the proteins was often part of a doctorate lasting several years.” Together with Andreas Terfort (Goethe University) and Andrey Turchanin (Friedrich Schiller University Jena), the idea evolved a few years ago of fishing the desired proteins directly out of mixtures by equipping a nanosheet with recognition sites onto which the target protein bonds. The researchers have now succeeded in making proteins directly available for examination using electron cryo-microscopy through a “smart nanosheet”.
Electron cryo-microscopy is based on the shock-freezing of a sample at temperatures under -150 °C. In this process, the protein maintains its structure, no interfering fixing and coloring agents are needed, and the electrons can easily irradiate the frozen object. The result is high-resolution, three-dimensional images of the tiniest structures – for example of viruses and DNA, almost down to the scale of a hydrogen atom.
In preparation, the proteins are shock-frozen in an extremely thin layer of water on a minute metal grid. Previously, samples had to be cleaned in a complex procedure – often involving an extensive loss of material – prior to their examination in an electron microscope. The electron microscopy procedure is only successful if just one type of protein is bound in the water layer.
The research group led by Turchanin is now using nanosheets that are merely one nanometer thick and composed of a cross-linked molecular self-assembled monolayer. Terfort’s group coats this nanosheet with a gelling agent as the basis for the thin film of water needed for freezing. The researchers then attach recognition sites (a special nitrilotriacetic acid group with nickel ions) to it. The team led by Rhinow uses the “smart nanosheets” treated in this way to fish proteins out of a mixture. These were marked beforehand with a histidine chain with which they bond to the recognition sites; all other interfering particles can be rinsed off. The nanosheet with the bound protein can then be examined directly with the electron microscope.
“Our smart nanosheets are particularly efficient because the hydrogel layer stabilizes the thin film of water required and at the same time suppresses the non-specific binding of interfering particles,” explains Julian Scherr of Goethe University. “In this way, molecular structural biology can now examine protein structures and functions much faster.” The knowledge gained from this can be used, for example, to better understand diseases and their treatment with drugs.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
EMA to relocate to Amsterdam - Working with Dutch government to ensure successful move by end of March 2019

Change of CEO at ZEISS in 2025 - Dr Karl Lamprecht will not extend his contract