Molecular glue degraders – a new weapon to target “undruggable” cancer drivers
One strategy to fight cancer is to target its Achilles' heel – the addiction to growth-promoting proteins. However, as many of these so-called cancer drivers are notoriously difficult to block with a drug, this requires novel and unconventional approaches. In cooperation with the German Cancer Research Center (DKFZ) and the National Center for Tumor Diseases (NCT) Heidelberg, researchers from Boston and Basel have elucidated the mechanism of action of one such unconventional drug. This “molecular glue degrader” binds to a cyclin-dependent kinase and induces its interaction with the cell's waste disposal system, which in turn results in degradation of a cancer driver and subsequent growth inhibition. The scientists' findings open up new paths in drug development.
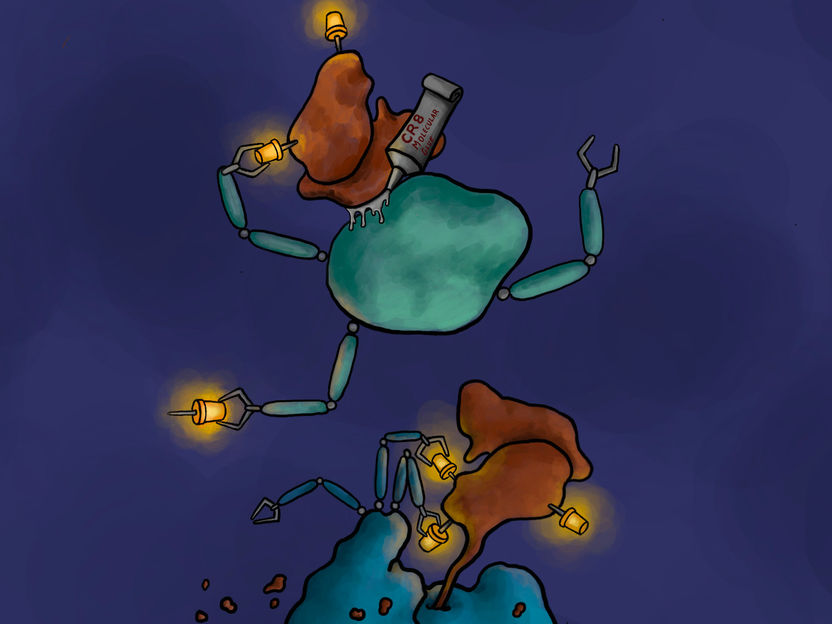
How a molecular molecular glue degrader works. The kinase inhibitor CR8 attaches the cancer-driving CDK12/cyclin K complex (brown) to an E3 ligase (green). This interaction causes cyclin K to be linked to ubiquitin molecules (yellow). Cyclin K molecules labeled in this way can be recognized and broken down by the cell's waste disposal system (blue).
Copyright: Jonas Koeppel
Most targeted cancer drugs currently used in the clinic are inhibitors, small molecules that work by binding to and blocking the activity of enzymes. However, only a fraction of proteins critical for cancer growth have a suitable pocket for inhibition. A novel approach to target such “undruggable” cancer drivers is to hijack the molecular machinery designed to remove aberrant proteins from the cell by degradation.
A vital component of the cell’s waste disposal system are E3 ligases, enzymes that regulate the abundance, and hence the activity of many proteins. In recent years, drugs were described that hijack E3 ligases to selectively destroy and remove disease-causing proteins. The best-known example is thalidomide, which acts as a “molecular glue” that induces proximity between a transcription factor called Ikaros and an E3 ligase substrate receptor. By “gluing” these two proteins together, Ikaros is labeled with ubiquitin and thereby targeted for degradation. A thalidomide analog called lenalidomide has been approved in Germany for the treatment of certain blood cancers, namely multiple myeloma and myelodysplastic syndrome.
“To identify new molecular glues, we investigated thousands of drugs for their ability to inhibit cancer cells in relation to the expression levels of E3 ligases", reports Mikołaj Słabicki, postdoctoral researcher at the Dana-Farber Cancer Institute (DFCI) in Boston and in the Division of Translational Medical Oncology at DKFZ and NCT Heidelberg and co-first author of the study. "We discovered that the kinase inhibitor CR8 inhibits the growth of cancer cells by recruiting an E3 ligase to the growth-promoting CDK12/cyclin K complex, which results in rapid degradation of cyclin K”.
Upon closer characterization of CR8, the researchers found a structural similarity to a known kinase inhibitor called roscovitine or seliciclib. “We have shown that it is possible to take a conventional kinase inhibitor and, by attaching a particular chemical group, transform it into a molecular glue degrader,” said co-senior author Benjamin Ebert, Chair of the Department of Medical Oncology at DFCI and recipient of the 2019 Meyenburg Cancer Research Award. The molecular details of the CR8-induced molecular glue complex were revealed by researchers from the Friedrich Miescher Institute for Biomedical Research (FMI) in Basel, including co-senior author Nicolas Thomä and co-first authors Zuzanna Kozicka and Georg Petzold. The teams on both sides of the Atlantic concluded that a small chemical moiety is required for CR8 to act as a molecular glue degrader. “This could be a more generalizable strategy for drug design and result in new options for the treatment of cancer”, says Kozicka.
The quest for novel molecular glue degraders was a joint effort by scientists from DFCI, the Broad Institute of Harvard and Massachusetts Institute of Technology in Cambridge, and FMI in collaboration with investigators at DKFZ and NCT Heidelberg, including two Major Cancer Biology students, Manisha Manojkumar and Jonas Koeppel.
“This study was true detective work, with every experiment uncovering additional pieces of the puzzle. It required a multi-disciplinary effort by team members from various fields, including cancer biology, bioinformatics, functional genomics, structural biology, and biochemistry, and was an incredibly exciting and rewarding experience”, says Słabicki.