Swedish Orphan Biovitrum has decided to move Kiobrina into phase III development
Swedish Orphan Biovitrum announced the results from the second Kiobrina® clinical phase II study. The study demonstrated an improvement in preterm infant growth velocity when Kiobrina® was administered in pasteurized breast milk. As a consequence of this outcome and the previously announced positive results from a phase II study in preterm infant formula, Swedish Orphan Biovitrum has taken the decision to move Kiobrina® into phase III development.
The combined results of the two clinical studies showed a statistically significant increase (p<0.001) in growth velocity, which is a medically relevant parameter. The safety profile was comparable to that of placebo and no drug-related serious adverse events were reported. The results from these studies will be published during 2010, starting with a presentation of the results from the first clinical study with infant formula at "The Power of Programming 2010. International conference on developmental origins of health and disease" in Munich, Germany on May 6-8, 2010.
"Kiobrina holds a great opportunity to fill a substantial medical need in neonatal care. The result from our phase II program is an important step towards a valuable product that will support preterm infants in their growth and development" said Peter Edman, CSO of Swedish Orphan Biovitrum Group.
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Celesio acquires Belgian wholesaler Laboratoria Flandria
BD Biosciences and StemCell Technologies Sign License Agreements with WARF - Commercialization of Novel Culture Media and Surfaces for Human Embryonic Stem Cell Research
Gellan_gum
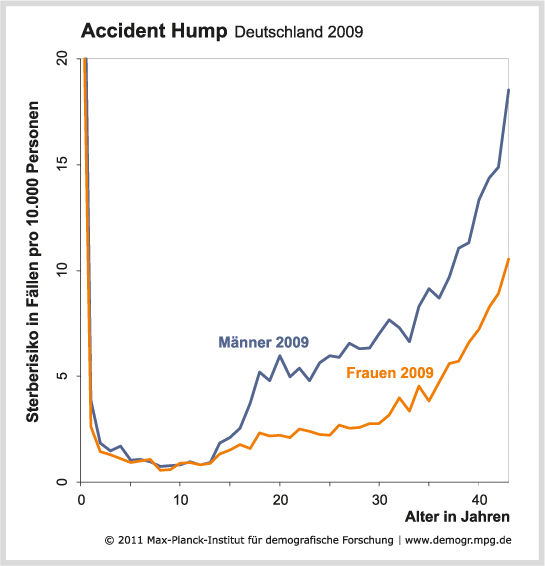
Boys reach sexual maturity younger and younger - The phase between being physically but not socially adult is getting longer
Shire's Vyvanse Will Attain Blockbuster Status by 2017 in the Attention-Deficit/Hyperactivity Disorder Drug Market - Emerging Nonstimulant Drugs Will Earn Less Than 15 Percent Share of the ADHD Market in 2017, According to a New Report from Decision Resources
CellCept reaches positive results in Phase III trial in Lupus Nephritis