CD4+ Cells in the Fast Lane
Effect of CAR-T Cells in Focus: Sub-group of T immune cells kill cancer cells significantly more effectively than CD8+ T cells
CAR-T cells are T lymphocytes, which exist naturally in the human body. They are genetically modified outside the body to equip them with a new function to kill cancer cells when they are returned to the patient. In current research studies in animal models, researchers of the Paul-Ehrlich-Institut, showed that a sub-group of T immune cells kill cancer cells significantly more effectively than those previously assessed to be the main actors, the CD8+ T cells. Unlike the authorised CAR-T cell medicinal poducts, the research team at the Paul-Ehrlich-Institut generates the CAR T cells directly in the organism.
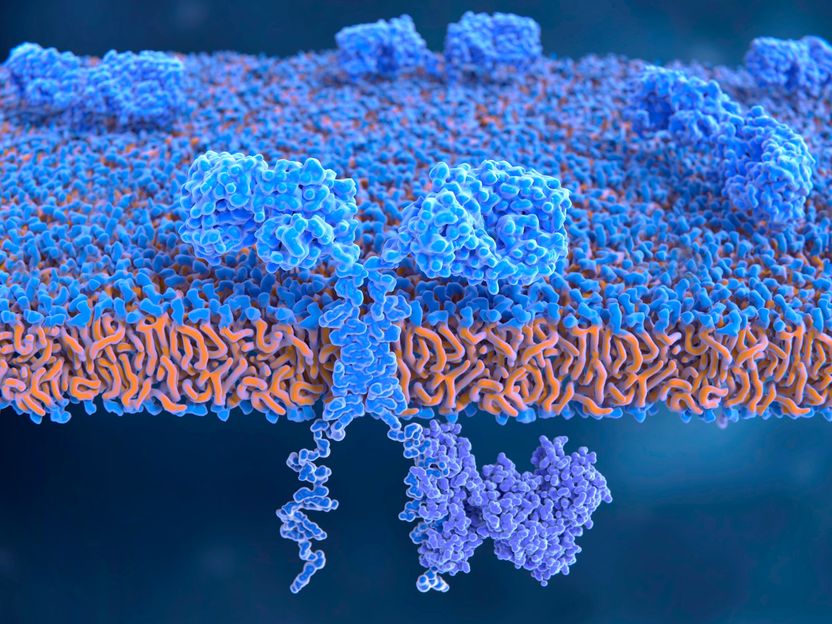
The chimeric antigen receptor (CAR) molecule integrated in the cell membrane of a T lymphocyte
Juan Gaertner
Major attention was paid to the marketing authorisation granted by the European Commission in 2018 for two so-called CAR-T cell gene therapy medicinal products. These are immune cells (T cells) from cancer patients equipped with a synthetic chimeric antigen receptor (CAR) outside the body using genetic engineering methods, propagated, and then returned into the patients’ body. The antigen receptor exactly matches the specific surface structures on the cancer cells. With the aid of the CAR, the immune cells recognise the cancer cells and kill them.
Thanks to their extraordinary effectiveness, CAR-T cells enjoy major interest: in certain leukaemia patients, where all therapies had previously failed, the cancer was no longer detectable after the CAR-T cell therapy. The manufacturing of these medicinal products – removal of the cells from the body, genetic modification outside the body, and reinfusion – however, proved to be very complex.
During the same year, scientists of the research team "Molecular Biotechnology and Gene Therapy" headed by Professor Christian Buchholz, succeeded in generating human CAR-T cells in mice in vivo – in other words, directly in the organism. The transfer of the genetic information for the formation of the CAR proved to be successful with specifically modified lentiviral vector particles (vectors), which transfer the CAR gene exclusively to particular T cell sub-types responsible for combatting the cancer.
When CAR-T cells are manufactured, both so-called CD4 positive (CD4+) and CD8-positive (CD8+) T cells are normally equipped with the chimeric receptor in the mixture. Up to now, it was assumed that, above all, CD8+ CAR T cells eliminate cancer cells. Now, funded by the German Cancer Aid and the LOEWE centre Frankfurt Cancer Institute, the research group was able to use their method to question a previous postulate. In their tumour mouse model with the human blood system, they used either exclusively CD4-specific or exclusively CD8-specific lentiviral vectors to create CD19-specific CAR T cells in the organism, which were thus targeted to tumour cells and B lymphocytes. The researchers (m/w/d) tested the activity of the CAR-T cells by measuring the decrease in tumour cells and in B lymphocyte levels. To their surprise, the CD4+ CAR-T cells showed an unexpectedly strong activity which was at least as pronounced as that of the CD8+ CAR-T cells. It can be assumed that this is because the state of exhaustion is reached less rapidly by CD4+ CAR-T cells, especially in the presence of large amounts of tumour cells, than is the case with CD8+ CAR-T cells.
"Our research results point to a more direct role of CD4-positive lymphocytes for the elimination of tumour cells in CAR-T cell therapy", as Professor Buchholz explained the current results. The latest results will possibly provide another module in generating specific and effective CAR-T cells directly in the patient. However, this will require further pre-clinical and, later on, additional clinical studies.





















































