A molecular pressure cooker tenderizes tough pieces of protein and helps to bite off
proteins are composed of amino acids connected by amide bonds. The amide bond exhibits high chemical stability and has a planar structure around the bond. Although the high stability of amide bond is indispensable to maintain protein functions, it is problematic to convert the building block into some other molecular species by selective dissociation of a relevant amide bond. There have been attempted to control reactivity of a specific amide bond with selective twisting of the bond by complicated chemical modifications. Some model compounds with twisted amide bonds have been produced by multi-step organic synthesis and their high reactivity has been demonstrated. It is presumed that the high reactivity of these twisted amide bonds is also used in vivo. Some proteins seem to be selectively cleaved by twisting specific amide bonds during autolysis and splicing. These proteins, unlike artificially synthesized model compounds, are supposed to use non-covalent interactions to twist their amide bonds. The researchers at the University of Tokyo and Institute for Molecular Science have fabricated for many years their molecular cages, which are self-assembled by the non-covalent interactions. They applied their molecular cages to confine amide molecules, which can be regarded as analogs of small pieces of proteins, and squeezed the amide bonds by pressurizing them inside their cage.
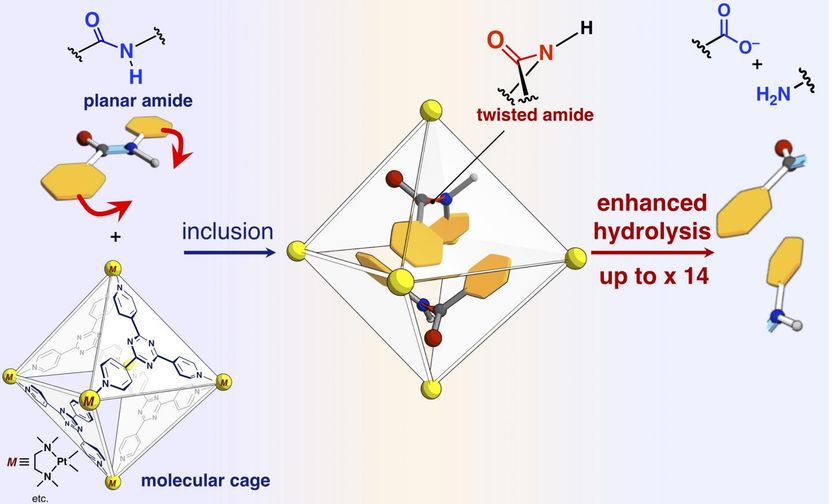
This is a scheme of reaction rate acceleration of amide hydrolysis by inclusion in the self-assembled molecular cage.
NINS/IMS
The researchers have reported in the present paper that amide bonds, which have planar structures and are inert in free space, can be twisted and the amide compounds can be activated by confining them into their molecular cage. When target amide compounds and the molecular cage are mixed and heated in an aqueous solution, the cage confines the amide compounds. Single-crystal X-ray structure analysis revealed that two amide compounds with twisted structures are confined in the cage. The twist angle around the amide bonds was found to reach 34 degrees. The reaction rate of hydrolysis of the twisted target was accelerated by a factor of five. The researchers succeeded in creating a new artificial enzyme of unexploited mechanism that it confines and twists the target molecules to activate a specific chemical bond.
The researchers also succeeded in altering the reactivity of target molecules by confining "stuffing molecules", which are not involved in the reaction, together with the targets in the cage, thereby precisely controlling the degree of twisting of the amide bonds. Without the stuffing molecule, the two of target amides are confined in one cage. One of the two targets is twisted and another one remains planar. In contrast, when conical stuff is mixed and then involved together with the target in one cage, the target remains planar. When a planar stuffing molecule is involved with the target, the stuff changes the shape of target into a twisted structure. The researchers investigated the reaction rates of hydrolysis in the two cases and found that the planar stuff (twisted target) accelerate the rate by 14 times, while the conical stuff (planar target) accelerated the rate by three times. The stuffing molecules allows us to tune the reaction rate precisely. This is an unprecedented achievement which has never been found in previous researches. This research gives us a novel method for the activation of inert molecules and can be applied to a variety of organic reactions.
The researchers showed that the amide molecules can be activated by twisting inside the cage without cumbersome chemical modification processes. "We are looking for new type of cages which can activate the targets with higher efficiency and apply them to other categories of target molecules. With our new cages, we will develop the novel activation method of inert molecules. In the future, our cages will be used as catalysts, which selectively squeeze and activate a specific bond of a target molecule and also as activation agents for prodrugs working in our body," said Fujita.