Molecular networks serve as cellular blueprints
Networks are at the heart of everything from communications systems to pandemics. Now researchers have found that a unique type of network also underlies the structures of critical cellular compartments known as membraneless organelles. These findings may provide key insights into the role of these structures in both disease and cellular operations.
Researchers have found that unique types of networks underlie the structures of membraneless organelles such as stress granules and processing bodies. Image from the researchers.
The researchers
"Prior to this study, we knew the basic physical principle by which these protein-rich compartments form - they condense from the cytoplasm into liquid droplets like dew on a blade of grass," said David Sanders, a post-doctoral researcher in Chemical and Biological Engineering at Princeton University. "But unlike dew drops, which are composed of a single component (water), cellular droplets are intimidatingly complex. Our work uncovers surprisingly simple principles that we think are universal to the assembly of liquid organelles, and opens new frontiers into studying their role in health and disease."
Sanders is the lead author in an article in the journal Cell describing a blueprint for the assembly of these liquid structures, also called condensates. The researchers looked closely at two types of condensates, stress granules and processing bodies ("P-bodies"). In the Cell paper, researchers directed by Clifford Brangwynne, a professor of Chemical and Biological Engineering at Princeton and the Howard Hughes Medical Institute, combined genetic engineering and live cell microscopy approaches to reveal the rules underlying the assembly and structure of stress granules, and why they remain distinct from their close relatives, P-bodies.
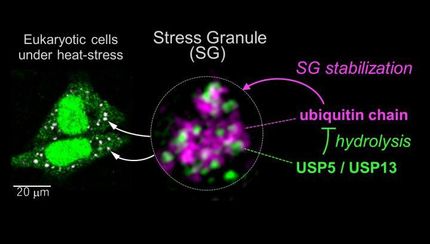
Stress granules earn their namesake by appearing when cells are removed from their comfort zone--for example, following heat or detection of noxious chemicals. Both stress granules and P-bodies are tied to an influx of RNA, genetic material that acts as the messenger between the cell's permanent genetic code (DNA) and its principal workhorses (protein). Although the function of these condensates is unclear, some scientists believe both play a role in supervising the cellular actions of the RNA messenger. Regardless of function, stress granules are hijacked by viral infections and have been implicated in diseases of aging. Understanding their assembly is thus critical to the development of new therapeutics.
In healthy cells, cellular machines called ribosomes continually move along the RNA assembly line, insulating the genetic message from its environment and manufacturing proteins essential for the cell's survival. When an external stress halts the assembly line, however, RNA is stripped bare and clumps together into stress granules and P-bodies. Brangwynne's team developed an approach to determine how this clumping occurs and why certain clumps prefer to associate, yet keep their components from mixing into a uniform droplet.
From previous studies by co-authors at Harvard University, the researchers knew a protein called G3BP, which is specifically targeted in many viral infections, is necessary for stress granules to form. But what makes G3BP special? To determine this, the researchers used gene-edited cells that lack G3BP and are unable to form stress granules following the addition of a chemical.
"This allowed us to add back components, one by one, to see what was necessary for their formation," Sanders said. "The first step was to add the G3BP back with various parts of the protein missing."
This approach identified two parts of the protein that were essential: one part that binds RNA and another which binds to specific proteins. Remarkably, the researchers were able to reverse engineer this molecular rescue by linking together similar parts from unrelated proteins. The team showed that stress granule protein scaffolds required a specific collection of building blocks, with a requisite number of chemical connectors that grabbed onto RNA strands and clumped them together. The connectors, called RNA-binding domains, are found on many types of proteins, but come in different flavors that specify their biological function.
"It was remarkably simple to reconstruct this," Sanders said. "All you needed was a scaffolding. Any RNA-binding domain would work as long as there were enough of them."
Eventually, the researchers were able to form identical condensates using more than a dozen different scaffolds. "What really mattered was whether there were enough binding domains to contact RNA and then connect them together in space," Sanders said. "Similar ideas have been used to understand the assembly of non-living materials, which inspired our thinking about the problem from a network perspective."
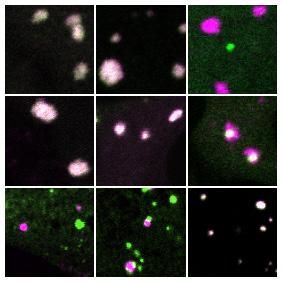
The researchers realized that the formation of the condensates was at heart a networking problem. When there were sufficient binding domains available to grab hold of RNA strands, the condensates rapidly formed. At the cellular level, concentrations of proteins with binding domains determined whether stress granules would or would not occur at any spot in the cell.
This understanding also provided insight about the well-known tendency of stress granules and P-bodies to stick together. The tendency, which is the subject of intense inquiry by numerous research groups, is related to the internal networks of stress granules and P-bodies, and the way those networks intersect.
"It just so happens that G3BP has the greatest abundance and network affinity of stress granule proteins," Sanders said. "It likes to interact with other proteins that similarly grab hold of RNA. The reason why stress granules and P-bodies stick together is that they overlap in their networks, forming an adhesive glue. What's interesting is that the two liquids have enough network overlap to stick together, but are different enough that they don't fully mix. If you increase the degree of network overlap, you can collapse the droplets into a single condensate. Moreover, if you remove the overlapping network glue, they detach. Thus, describing condensates as stress granules or P-bodies is really a false dichotomy. What matters is network connectivity and this will be determined by distinct cellular states."
Brangwynne shared his excitement about future work building from these studies, determining whether the blueprint for stress granules and P-bodies applies to other condensates such as nucleoli. "Our group had previously shown that nucleoli exhibit a similar structure, like different types of oil droplets that do not mix, all floating within a water solution. But we didn't understand the molecular rules. Using the stress granule system, we may have uncovered a general framework for understanding this problem, which is exciting to think about in the context of different areas of biology, and for exploiting in biomedical applications such as organelle engineering and therapeutics."