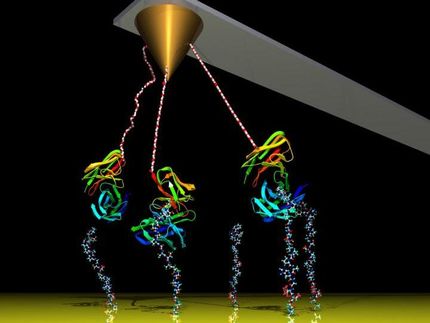
Lifting the lid on beta-barrels
The interaction between biotin and streptavidin is a well-established experimental tool in bionanotechnology. LMU physicists have now shown that the mechanical stability of the complex is dependent on the precise geometry of the interface.
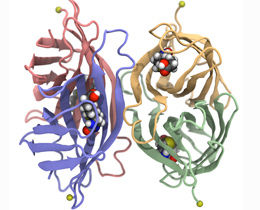
Streptavidin is made up of four identical subunits (shown here in green, blue, yellow and red), each of which can bind to a biotin molecule (colored spheres). In the experiment, streptavidin is fixed to an anchor (yellow sphere) while a biotin molecule is pulled out of one of the binding pockets.
Rafael C. Bernardi
Mechanical forces play a vital role at all levels in biological systems. The contraction and relaxation of muscle cells is undoubtedly the best known example of this, but mechanosensitive proteins are actually found in virtually all cells. For example, the shear stress exerted by blood flow on the cells that line blood vessels is sensed by mechanoreceptors, which trigger signaling pathways that control vessel diameter. In their efforts to understand the molecular mechanisms that mediate such processes, scientists study the responses of mechanosensitive proteins by analyzing their behavior under mechanical stress. Many of these experiments rely on the use of the tight and highly specific interaction between biotin (a vitamin) and its binding protein streptavidin as a force gauge. In collaboration with Rafael Bernardi at the Beckman Institute in Urbana (Illinois), LMU biophysicists led by Professor Hermann Gaub have now performed a detailed analysis of the mechanical stability of this complex, which appears in the journal Science Advances. Their findings show that the geometry of the interface between the ligand and its binding pocket in the receptor has a marked impact on the stability of the complex, and that this factor must be taken into account when evaluating experimental data.
A molecular dowel
Physicists use a technique called single-molecule force spectroscopy to measure how biomolecules react to mechanical forces. In this method, the protein of interest is bracketed between two molecular tags. One of these serves to covalently attach the protein to a glass slide. The other is linked to a setup that enables the experimenter to exert a graduated force on the protein. Often, this second linker makes use of the non-covalent interaction between biotin and streptavidin. “This ligand-receptor system is the ‘rawlplug’ of force spectroscopy,” says Steffen Sedlak, a member of Gaub’s team and lead author of the paper.
The biotin tag is covalently attached to the protein itself and is thus available for high-affinity binding to streptavidin. The remarkable mechanical stability of the interaction between the ligand and its receptor is a vital factor in these experiments. Since the first force spectroscopy experiments on this system by Gaub some 25 years ago, measurements of the response of the complex to mechanical forces have been measured by force spectroscopy in laboratories all over the world. However, the results obtained in different settings have been inconsistent and have shown quite a wide range of variation. The new study set out to determine the reasons for this.
The streptavidin protein is made up of four structurally similar domains, named ‘beta-barrels’ because they are comprised of parallel strands that are arranged like the staves of a cask. Each of these barrels can accommodate one biotin molecule – and like every self-respecting barrel, each is equipped with a lid, which closes over the bound biotin. In this conformation, the biotin is accessible only from the other end – the end that is attached to the protein of interest.
Extracting biotin from its barrel
For their experiments, Sedlak and his colleagues created several mutant variants of the streptavidin protein. In each case, only one specific barrel structure was capable of binding to biotin, while the other three remained empty. To measure the stability of binding to each individual barrel, the team measured the forces required to pull the bound biotin out of the four streptavidin proteins. “We discovered that the magnitude of the force required to extract the biotin varied depending on which of the four barrels it was sitting in – even though all four of the active binding pockets are exactly the same,” Gaub explains.
Out the door or through the wall?
With the aid of complex computer simulations, the team was able to puzzle out the reason for these surprising findings. When subjected to a gradually increasing mechanical force, the biotin molecules in the different pockets apparently take up different positions before the force becomes sufficiently large to displace them. This in turn alters the geometry of subsequent force application, and this factor can account for the differences in the response of each pocket. Extraction occurs most readily when the force is able to act on the flexible lid of the beta-barrel, but if tension is exerted on any other position, the system can resist much higher stresses. “As Diogenes himself surely discovered, it’s easier to exit from a barrel by lifting the lid than by loosening the staves,” says Sedlak. “But the fact that this principle also holds for the action of mechanical forces on single biomolecules is not at all trivial.”
The authors believe that these results will make it possible to optimize streptavidin for use in force spectroscopy experiments – by allowing experimenters to tune the stability of the dowel’s position in the barrel.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!