Traumatic brain injury: designer molecule supports brain repair
International research team has discovered how repair after a brain injury can be improved by influencing immune cells
Advertisement
With a traumatic brain injury, a varying number of nerve cells in the brain die off, depending on the severity of the injury. Such brain injuries can impair someone’s concentration, the ability to make decisions, learning and memory. A molecule developed by Professor Stefan Rose-John, member of the Cluster of Excellence "Precision Medicine in Chronic Inflammation" (PMI), and Executive Director of the Institute of Biochemistry at Kiel University (CAU), could trigger a mechanism that repairs this damage, as indicated by new research results which Rose-John and international researchers under the leadership of the University of Queensland in Brisbane, Australia recently published in the scientific journal Cell.
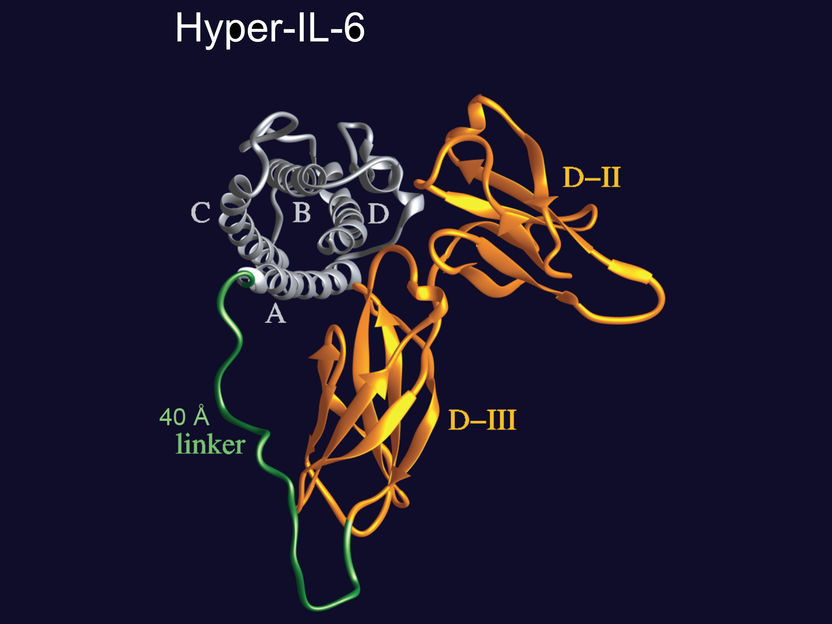
With the designer molecule "Hyper-IL-6", an IL-6 molecule is firmly bound to its specific soluble receptor. The new molecule can thus activate the IL-6 trans-signaling pathway much more effectively than both molecules independently.
© S. Rose-John, Uni Kiel
The researchers investigated how the immune system in the brains of mice interacts with brain nerve cells after an injury, and how this influences the memory and the ability to learn. "Previously, we assumed that after a brain injury, specific immune cells in the brain, the microglia, promote inflammation of the brain and thereby lead to a reduction in cognitive skills," reported the lead author of the publication, Dr. Jana Vukovic, from the University of Queensland. "However, when we removed microglia from mice we were surprised that there was absolutely no change in their behaviour or ability to repair brain tissue," continued Vukovic. In response to this finding, the research team then stimulated increased formation of new microglia in animal experiments and discovered that this promotes the repair of nerve cells. "The rejunevated microglia improved the mice's learning an memory, preserved tissue loss and stimulated the birth of neurons," said Vukovic.
Interleukin-6 promotes repair
The driving force behind this regenerative process is the signaling molecule Interleukin-6 (IL-6): when the researchers blocked it completely in experiments, the regenerative effect of microglia also disappeared. When they increased the amount of IL-6 present, they stimulated the process. The signaling molecule is an important chemical messenger which raises the alarm if inflammatory reactions occur, for example, when it is secreted in increased quantities, and thus regulates immune responses. It can function via two different signaling pathways: in the "traditional" signaling pathway, IL-6 specifically binds to a receptor which is only present in certain cells, such as liver cells. Together, they then bind to another receptor subunit on the same cell, the so-called gp130 protein, thereby triggering a reaction in the cell. In the alternative so-called "IL-6 trans-signaling pathway", on the other hand, IL-6 can affect every cell in the body under certain circumstances. The specific IL-6 receptor is also present in soluble form in the blood. IL-6 can bind to these free receptors circulating in the bloodstream. This compound then binds to a gp130-receptor, which is present on all cells, and triggers a reaction in the cell.
A designer molecule as a molecular diagnostic tool
Rose-John discovered this trans-signaling pathway, which is involved in many physiological processes, and has achieved pioneering research in investigating its central importance. "We have developed several molecular tools over the past few years that we can use to test which signaling pathway is active in a particular disease pattern," explained Rose-John. These molecular tools include the artificial molecule "Hyper-IL-6", which was also used in the current study. It consists of the signaling molecule Interleukin 6 (IL-6) and its specific receptor in soluble form, i.e. not bound with a cell. Together, they form a completely new molecule which does not exist in this form in nature. Just like its naturally-occurring individual components, this new molecule also stimulates the IL-6 trans-signaling pathway, but it is significantly more effective since IL-6 and its receptor no longer have to "find each other" in the blood, like they would normally do in nature.
Regeneration via the IL-6 trans-signaling pathway
In the current study, Hyper-IL-6 showed a positive effect: after administering the molecule to injured nerve cells, more microglia formed, which in turn led to the formation of new nerve cells, and ultimately to an improvement of the symptoms. "The results show that the newly-discovered regenerative effect of microglia after a brain injury is triggered by IL-6 via the trans-signaling pathway," said Rose-John. "This observation is also interesting, because up until now we had no idea how IL-6 works in the brain."
In future, the results could enable the development of new drugs to alleviate learning and memory deficits after the nerve cells have been damaged. They therefore offer promising potential for treating a variety of neurological disorders such as brain injury, dementia and other neurodegenerative diseases.