Loss of Protein Disturbs Intestinal Homeostasis and Can Drive Cancer
An international team of researchers from the University of Zurich, the University Hospital Zurich, Heidelberg and Glasgow has identified a novel function for the cell death regulating protein MCL1: It is essential in protecting the intestine against cancer development – independent of bacterial-driven inflammation. These findings have implications for the use of MCL1 inhibitors, currently being tested for cancer treatment.
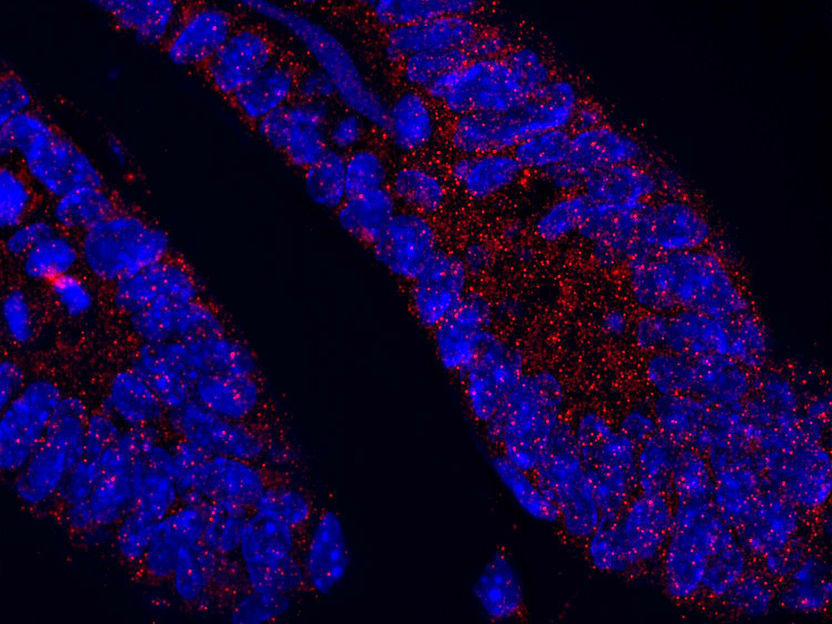
The protein MCL1 (red dots) within the small intestine of a healthy mouse. The nuclei of individual cells (blue) are also visible.
Marc Healy, UZH
Colorectal carcinoma (CRC), the most common form of intestinal cancer, is the second leading cause of cancer related death worldwide. While some patients have a genetic predisposition to the disease, the majority of cases are sporadic and largely influenced by the ever-increasing “Western lifestyle”, which includes obesity, poor diet and physical inactivity.
A recently published study now sheds new light on how this disease develops: Through the use of genetically modified mice, an international team of researchers was able to demonstrate that the protein MCL1 is essential for maintaining intestinal homeostasis and thus protecting against intestinal cancer formation. The research project was led by Achim Weber, professor at the Institute of Molecular Cancer Research at the University of Zurich and at the Institute of Pathology and Molecular Pathology of the University Hospital Zurich, in collaboration with researchers from the German Cancer Research Center in Heidelberg and the Beatson Institute in Glasgow, Scotland.
MCL1 protein protects the intestine against cancer
For their study, the researchers modified the genetic makeup of mice so that the animals’ intestinal cells would no longer produce the MCL1 protein. This protein normally prevents the death of cells and thus maintains the right balance of dying and new cells in the intestinal mucosa. The loss of MCL1 resulted in irreparable damage to the intestine and the subsequent formation of intestinal tumors. Similar changes can also be observed in the intestine of humans suffering from chronic intestinal inflammation, who also carry an elevated risk of developing intestinal cancer.
Tumors also develop without bacterial-driven inflammation
Microbiota-driven chronic intestinal inflammation has long been considered essential in the development of intestinal cancer. “What’s remarkable, however, is that the loss of MCL1 can drive intestinal cancer even without bacteria-driven inflammation,” says Weber. This was demonstrated by experiments in which mice without the MCL1 protein were held in a germfree environment. “This means that the loss of certain genes is apparently enough to cause intestinal cancer – even in the absence of inflammation. This groundbreaking finding significantly furthers our understanding of the critical early steps associated with intestinal cancer development,” says Weber.
Treating cancer with MCL1 inhibitors is like walking a tightrope
This study also reveals a second surprising result: In some types of tumor – including colorectal carcinoma – there is too much MCL1, rather than too little. Researchers assume that these tumors ramp up the production of MCL1 to gain an advantage for survival and enable them to better resist conventional treatment methods. As a result, a number of new therapies are currently being trialled to interfere with and reduce MCL1 function.
However, the study’s findings show that not only the overexpression, but also the loss of MCL1 can be detrimental. It is possible that the loss of MCL1 function – even only temporarily – may trigger a disturbance of the intestinal mucosa and the initial steps of tumor development. “The regulation of this protein is like walking a tightrope,” warns Marc Healy, first author of the study. “Our study therefore urges an element of caution when it comes to using MCL1 inhibition in cancer therapy.”



















































